|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME Faculty of Natural Science, Department of Physics, Self-assembly and Self-organization Research Group
Supervisor: Dr. Lagzi István László
Time-varying pH chemical systems (clock reactions and pH oscillators) and their application in self-assembled reactions
Introducing the research area
Our research work can be divided into two parts: with the help of clock-type reactions with time-increasing pH, we realize the self-assembly of building blocks, and secondly, we drive (even biochemical) systems with pH-oscillating reactions, that can also be monitored by absorption spectrophotometry. [1] [2] [3] [4]
Brief introduction of the research place
The BME Self-Assembly and Self-Organization Research Group has long been involved in the studies and applications of the influence of oscillating chemical reactions [5] [6] [7] [8] (e.g., bromate-sulfite-bicarbonate or Belousov-Zhabotinsky reaction) and clock-type reactions [9] [10] (e.g., urea-urease, methylene glycol-sulfite) known from the literature on temporal and/or spatial self-assembly (e.g., in the production of gold nanoparticles, formation of oleic acid vesicles (Figure 1.) [2] [11], zeolitic imidazolate frameworks).
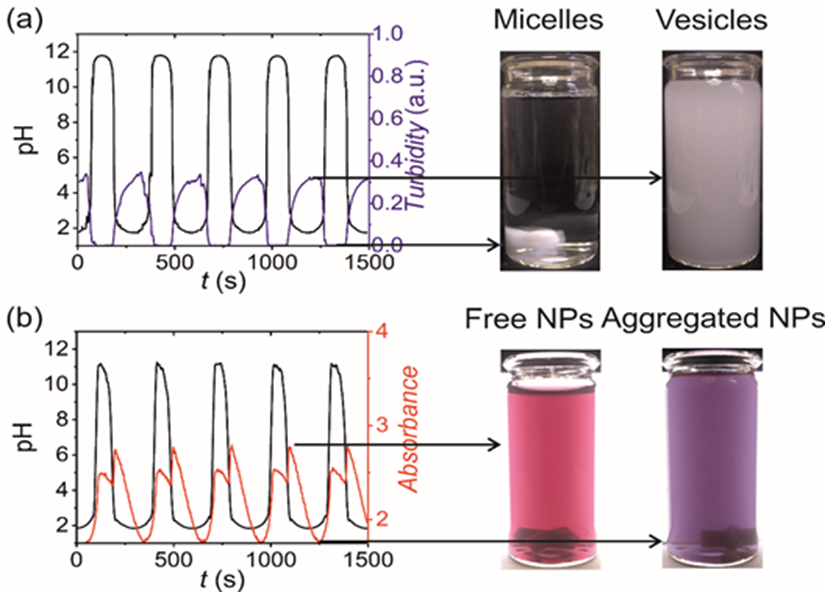
Figure 1. Application of an acid-base oscillating system to engineer (a) oleic acid vesicles and (b) aggregated gold nanoparticles in time. [2]
History and context of the research
Oscillating (also known as periodic) phenomena have long been known in physics, biology, and astronomy. Let's consider only the movement of the pendulum or the electric vibrating circuits. However, there are chemical reactions in which a change in some properties (such as hydrogen ion concentration) over time shows a temporal oscillation phenomenon (Figure 2. (a)). The best-known example is the Briggs-Rauscher reaction (also known as the iodine clock), the color change of which is visible to the naked eye or can be recorded spectrophotometrically. Another example is the Belousov-Zhabotinsky reaction, where the redox potential can be monitored by a computer using a calibrated glass electrode. Chemical systems in which the pH initially starts from low pH and shows a steep increase after a certain time (induction time) are called pH clock reactions (Figure 2. (b)) (such as the degradation of urea by urease enzyme which follows Michaelis kinetics or the sulfite-hydrogensulfite-methylene glycol reaction).
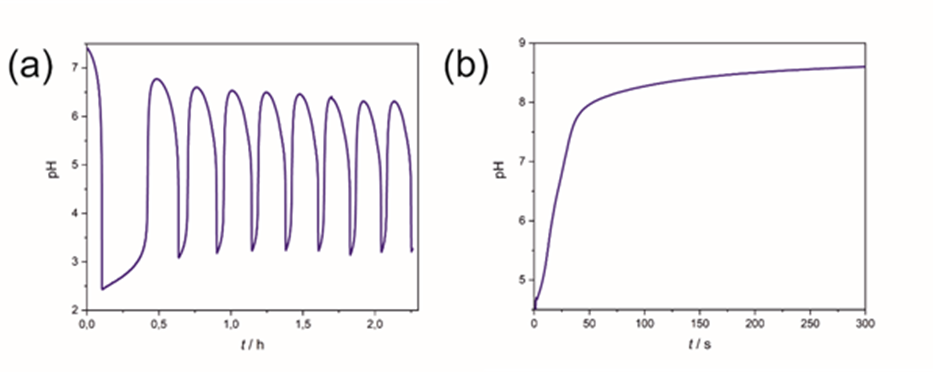
Figure 2. (a) Bromate-sulfite-bicarbonate pH oscillator and (b) urea-urease enzyme clock reaction
During self-assembly, the building blocks (molecules, frameworks, nanoparticles, etc.) due to their individual properties (e.g., coordination, van der Waals, etc.) form spontaneous structures after reaching a critical value and the system is far from thermal equilibrium. For example, metal-organic frameworks (MOFs) or gold nanoparticles can be prepared through self-assembly. There are many examples in the literature for the coupling of the pH oscillators and clock reactions, for example in the case of complex-, precipitation- and aggregation reactions. [8] [11]
The research goals, open questions
The main motivations for the measurements and research: usage of pH oscillators and clock reactions (which can be found in the literature, but in slightly modified form) to produce new material structures by self-assembly, and the direct or indirect coupling of systems that cannot oscillate on their own with pH oscillators.
To achieve this, it is necessary to design stable and reproducibly operating oscillating systems and to gain experience in discovering the possible catalytic or inhibitory properties of pH-sensitive building blocks on clock reactions or oscillators.
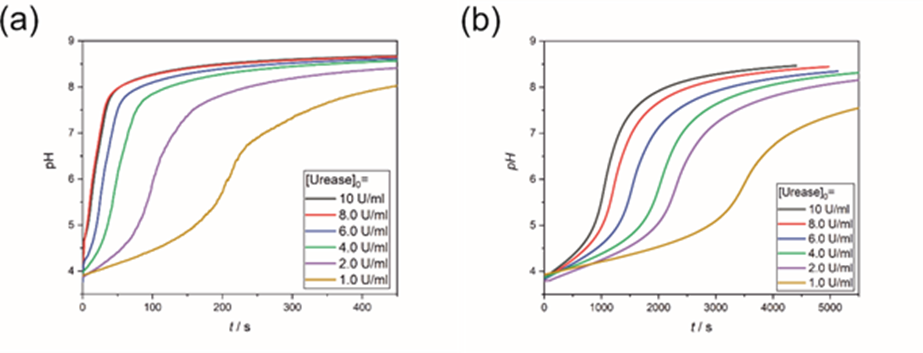
Figure 3. Investigation of the urea-urease enzyme clock reaction free (a) and in the presence of 2-Methylimidazole (b) with different amounts of the enzyme. [3]
The autocatalytically changing pH environment may provide an opportunity to develop new, individual morphology for MOFs, which are excellent materials for safer gas storage and separation due to their porosity and high thermal stability, as well as organic chemical catalysis due to their transient metal content.
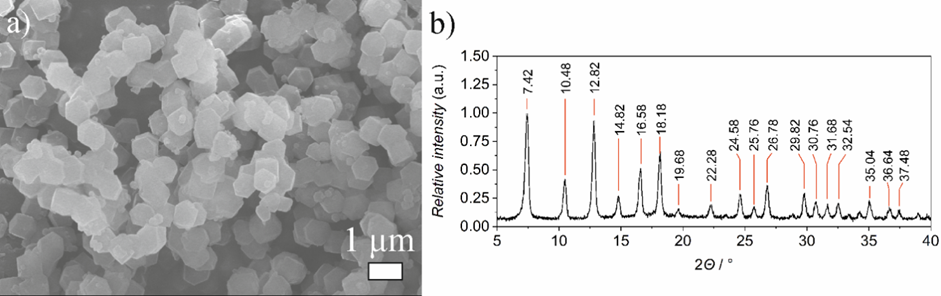
Figure 4. Scanning electron microscope micrograph (a) and X-ray diffraction spectra (b) of ZIF-8 metal-organic framework prepared by using clock reaction. [4]
Methods
In the case of clock-type reactions, stock solutions of the components in high-purity distilled water are pumped in a transparent continuously stirred reactor (CSTR), the initial pH is adjusted, and then the reaction is started by adding the catalyst solution. The pH is monitored using a platinum glass electrode and the turbidity change of the solution will be followed spectrophotometrically over time at a given wavelength. The solid precipitation from the reaction mixture will be filtered or centrifuged, washed several times, dried and finally scanning electron microscopic (SEM) micrographs and/or X-ray diffraction spectra will be taken to reveal the crystal structure.
In the production of stable pH oscillators, aqueous solutions of the components will be continuously stirred and thermostated into a tank reactor using a peristaltic or programmable pump at a defined flow rate, while the pH change will also be digitally monitored over time using a glass electrode. Another peristaltic pump will be used to drain excess liquid from the reactor to keep the constant volume there. It is important to choose the right flow rate during the addition of the components, as this will determine the residence time in the reactor. Stock solutions are freshly prepared before each measurement because some components (e.g., sodium sulfite) are degradable in the longer term in water. The gases periodically produced by the pH oscillators (carbon dioxide, carbon monoxide) are captured in a solution flowing through a thin-walled silicone tube (with the help of another peristaltic pump). The color change of this solution is monitored spectrophotometrically at a given wavelength in a flow-through cuvette. (Figure 5.) [1]
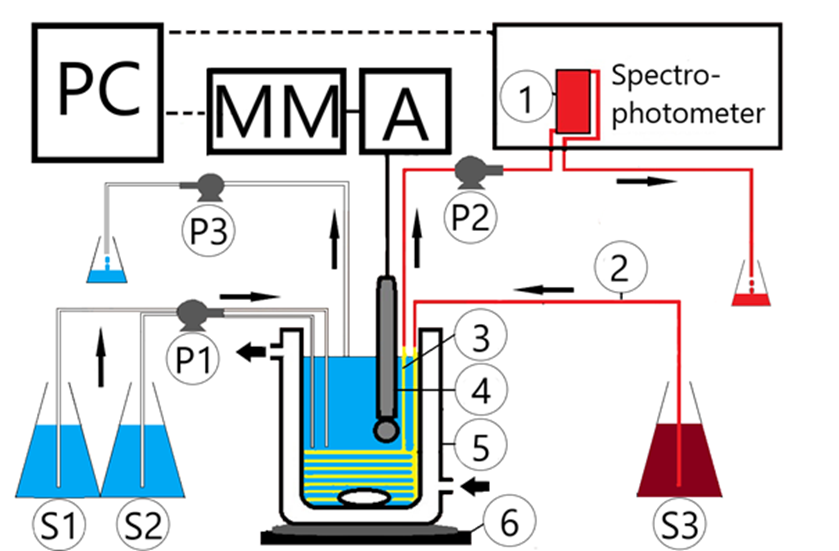
Figure 5. Coupling of a carbon dioxide-producing pH oscillator with a methyl red indicator system [1]
1: flow-through quartz cuvette, 2: Tygon tube system for methyl red solution flow, 3: permanent silicone tube for switching the pH oscillator and the methyl red solution system, 4: pH-sensitive glass electrode, 5: thermostated continuous stirred tank reactor (CSTR), 6: magnetic stirrer, P1-3: peristaltic pumps for flowing S1-3 stock solutions, A: amplifier, MM: multimeter, PC: computer
Some clock reactions in which self-assembly occurs can be later developed into constantly feed pH oscillators as long as the components do not inhibit the progress of the reaction too much.
Results
Over the last year and a half, it has been possible to create pH-oscillators with constant feeding methods (e.g. bromate-sulfite-bicarbonate, methylene glycol-gluconolactone-sulfite pH-oscillator) and to study their wave properties several times. In the case of bromate-sulfite-bicarbonate, the oscillation system was successfully connected to a silicone-Tygon tube system containing methyl red indicator solution (Figure 5.) so the oscillation could also be monitored spectrophotometrically.
Clock reactions (urea-urease, sulfite-hydrogensulfite- methylene glycol) were studied in the presence of ZIF-8 (zeolite-type metal-organic framework) components (zinc cation and 2-methylimidazole) and reaction kinetic results were obtained on their inhibition and precipitation. (Figure 3.)
Expected impact and further research
As our research progresses, pH-sensitive building blocks (e.g. gold nanoparticles, components of metal-organic frameworks (MOFs) will self-assemble, even to form new nanostructures in a time-increasing or oscillating chemical environment, without human intervention. In the case of the coupling of pH oscillators that periodically produce gas, the implementation of a biochemical sensor (activated hemoglobin solution) instead of the methyl red indicator stock solution is also included in our plans.
Publications, references, links
List of corresponding own publications.
[1] Német, N., Holló, G., Lagzi, I. Carbon Dioxide-Driven Coupling in a Two-Compartment System: Methyl Red Oscillator. J. Phys. Chem. A 124, 10758–10764 (2020) https://doi.org/10.1021/acs.jpca.0c09632.
[2] Lawson, H. S., Holló, G., Német, N., Teraji, S., Nakanishi, H., Horvath, R., Lagzi, I. Design of non-autonomous pH oscillators and the existence of chemical beat phenomenon in a neutralization reaction. Sci Rep 11, 11011 (2021) http://www.nature.com/articles/s41598-021-90301-8.
[3] Német, N., Ylenia, M., Schuszter, G., Tóth, L. E., Maróti, E. J., Szabó, P. J., Rossi, F., Lagzi, I. Inhibition of the urea-urease reaction by the components of the zeolite imidazole frameworks-8 and the formation of urease-zinc-imidazole hybrid compound. React Kinet Mech Cat 135, 15–28 (2022) https://link.springer.com/article/10.1007/s11144-021-02139-w.
[4] Német, N., Holló, G., Schuszter, G., Horváth, D., Tóth, Á., Rossi, F., Lagzi, I. . Application of a chemical clock in material design: chemically programmed synthesis of zeolitic imidazole framework-8. Chem. Commun. 58, 5777-5780 (2022) https://doi.org/10.1039/d2cc01139e
Table of links.
Webpage of the BME Self-Assembly and Self-Organization Research Group
Videos:
Briggs Rauscher reaction-Video
Belousov-Zhabotinsky reaction-Video
List of references.
[5] Orbán, M., Kurin-Csörgei, K., Epstein, I. R. pH-Regulated Chemical Oscillators. Acc. Chem. Res. 48, 593–601 (2015) https://pubs.acs.org/doi/10.1021/ar5004237.
[6] Rábai, G., Orbán, M., Epstein, I. R. Design of pH-regulated oscillators. Acc. Chem. Res. 23, 258–263 (1990) https://doi.org/10.1021/ar00176a004.
[7] Kovacs, K., McIlwaine, R. E., Scott, S. K., Taylor, A. F. pH oscillations and bistability in the methylene glycol–sulfite–gluconolactone reaction. Phys. Chem. Chem. Phys. 9, 3711–3716 (2007) http://www.nature.com/doifinder/10.1038/nature03214.
[8] Kurin-Csörgei, K., Epstein, I. R., Orbán, M. Systematic design of chemical oscillators using complexation and precipitation equilibria. Nature 433, 139–142 (2005) https://www.nature.com/articles/nature03214
[9] Hu, G., Pojman, J. A., Scott, S. K., Wrobel, M. M., Taylor, A. F. Base-Catalyzed Feedback in the Urea−Urease Reaction. J. Phys. Chem. B 114, 14059–14063 (2010) https://pubs.acs.org/doi/10.1021/jp106532d.
[10] Warneck, P. The formaldehyde-sulfite clock reaction revisited. J. Chem. Educ. 66, 334 (1989) https://pubs.acs.org/doi/abs/10.1021/ed066p334.
[11] Tóth S., E., Horváth, J., Holló, G., Szűcs, R., Nakanishi, H., Lagzi, I. Chemically coded time-programmed self-assembly. Mol. Syst. Des. Eng. 2, 274–282 (2017) http://xlink.rsc.org/?DOI=C7ME00020K.
