|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Chemical
Supervisor: Dr. Keglevich György
Green Synthesis and Cytotoxic Activity of α-hydroxyphosphonates and Related Derivatives
Introducing the research area
α-Hydroxyphosphonates have attracted attention due to their biological activity. They are widely known as enzyme inhibitors1, bactericides2,3, herbicides4 and antioxidants5,6. Owing to their wide range of bioactive effects, the synthesis of α-hydroxyphosphonates and related derivatives is an evergreen research area. During the last decades, environmental protection became one of the most significant factors in chemical industry. Along with this ambition, recent papers on α-hydroxyphosphonates also put much effort on the green chemical aspects of the synthesis7.

Brief introduction of the research place
The Green Chemical and Organophosphorus Research Group operating at the Department of Organic Chemistry and Technology led by professor Dr. György Keglevich is engaged with organophosphorus and green chemistry. The main fields of our interest involve the elaboration of green synthetic methods, the application of the microwave (MW) technique in organic chemical reactions, the optimization of reactions and the synthesis of new organophosphorus compounds.
History and context of the research
The most common synthetic route towards α-hydroxyphosphonates is the addition of dialkyl phosphite to an oxo compound. The discovery of this reaction dates back to the early 1950s8. At that early stage, the so-called Pudovik reaction was carried out in the presence of expensive and strong bases under harsh reaction conditions.
In the spirit of green chemistry, recent articles targeted the solvent-free synthesis of α-hydroxyphosphonates by avoiding the use of organic solvents during the chemical reaction9-12. However, the solvent consumption of the reaction was just the “tip of the iceberg” in these cases (Figure 1), the need for solvents during the purification was disregarded. In the procedures reported in the literature, α-hydroxyphosphonates were purified by a combination of column chromatography, extraction and recrystallization that all consume a considerable amount of volatile organic solvents. Despite the solvent-free reaction conditions, the reported protocols do not meet the requirements of green chemistry9-12. From an industrial point of view, this is a major issue to be handled considering environmental and economic aspects.
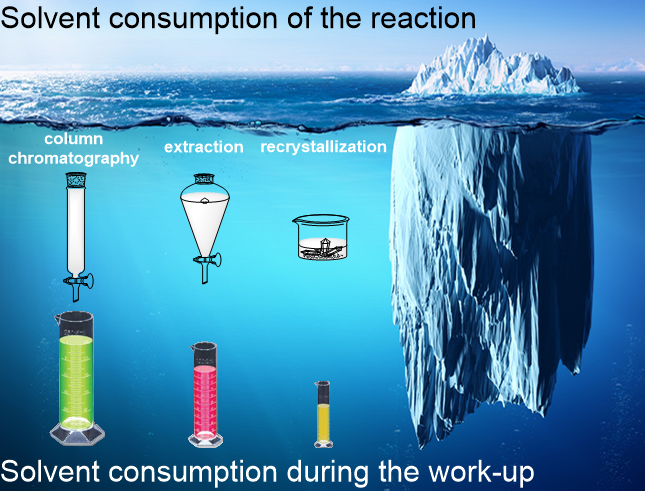
Figure 1. The amount of organic solvents in a chemical reaction represents the “tip of the iceberg”. The solvent consumption of the work-up is often more determinative.
The research goals, open questions
First, we aimed at the rationalization of the synthesis of α-hydroxyphosphonates from a green chemical approach. Our main target was to reduce the use of organic solvents in the chemical reaction as well as during the work-up.
Our next goal was the utilization of the α-hydroxyphosphonates so-obtained in a series of transformations to afford new, potentially bioactive compounds. The first target molecules were α-aminophosphonates (Scheme 1, Route B). The synthesis of α-hydroxyphosphonic acids (Scheme 1, Route C) and O-phosphoryloxyphosphonates (Scheme 1, Route D) were also involved in our research plan. Beside the development of new reaction routes, we were also curious to explore the mechanism of the chemical reactions.
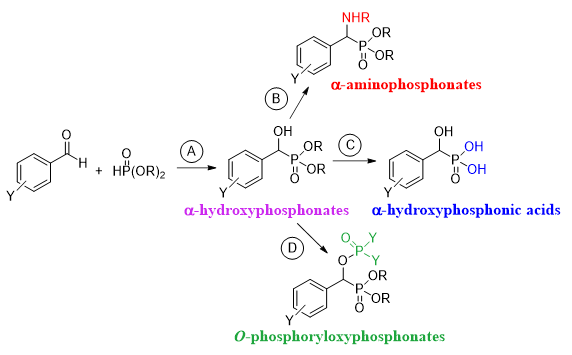
Scheme 1. Synthesis plan to obtain α-hydroxyphosphonates and related derivatives.
Despite the wide range of bioactive effects of α-hydroxyphosphonates, their anticancer effect has been barely studied13. We aimed at the cytotoxic assay of the derivatives obtained by us against a sarcoma cell line.
Methods
The synthesis of α-hydroxyphosphonates was carried out starting from substituted benzaldehydes and dialkyl phosphite as shown on Scheme 1, Route A. Triethylamine (Et3N) as a simple, inexpensive, organic base was used as the catalyst. According to our new procedure, a mixture of the starting components and the catalyst was stirred in a minimal amount of acetone at reflux. After the appropriate reaction time, pentane precipitant was added to the mixture. On cooling, the desired product crystallized out, and the α-hydroxyphosphonates were obtained by a simple filtration in high purity, eliminating the need for further purification (Figure 2)R1,R3. Despite the minimal use of organic solvent during the reaction, the total solvent consumption of the procedure could be significantly reduced.
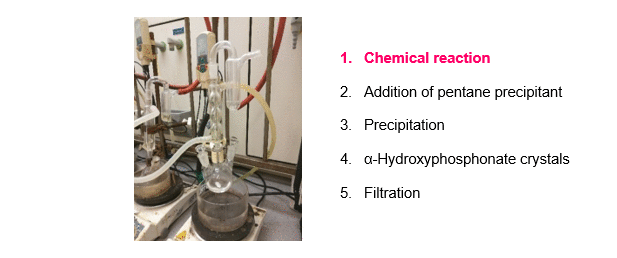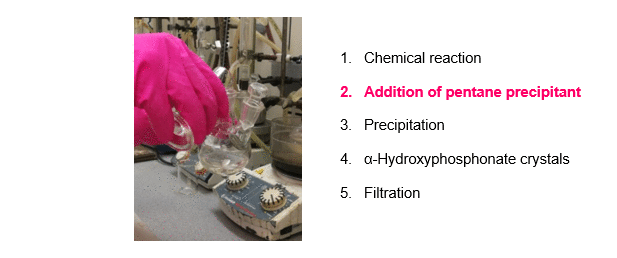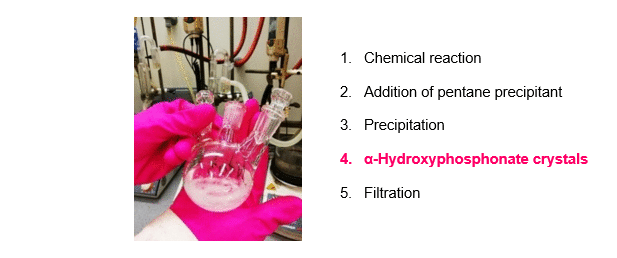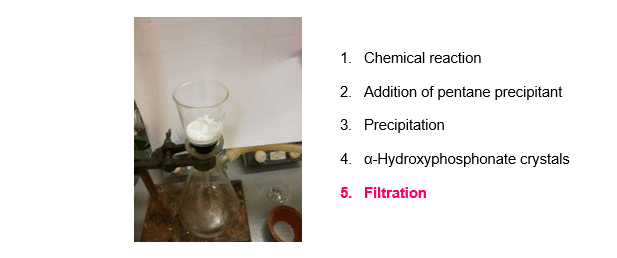
Figure 2. A new, green method for the synthesis of α-hydroxyphosphonates.
The crystal structure of α-hydroxyphosphonates was investigated by single crystal X-ray diffraction. This analytical method is based on the phenomenon that X-rays are diffracted from the electrons of the measured molecules and gives us an insight into the exact construction of the crystal-lattice.
α-Aminophosphonates were synthesized in the reaction of α-hydroxyphosphonates and primary amines under MW irradiation (Scheme 2)R2.

Scheme 2. Synthesis of α-aminophosphonates under MW conditions.
MW reactors work similarly to kitchen MW ovens. Traditionally, organic reactions are carried out on conventional heating, where heat transfer slows down the heating dramatically. However, in case of MW irradiation, the MWs interact directly with the molecules and cause their oscillation. The friction of molecules on one another leads to the formation of “hot spots”, where the temperature is higher than in the bulk of the reaction mixture (Figure 3). This phenomenon is called “superheating” and may open the gate for reactions that cannot be performed on conventional heating14.
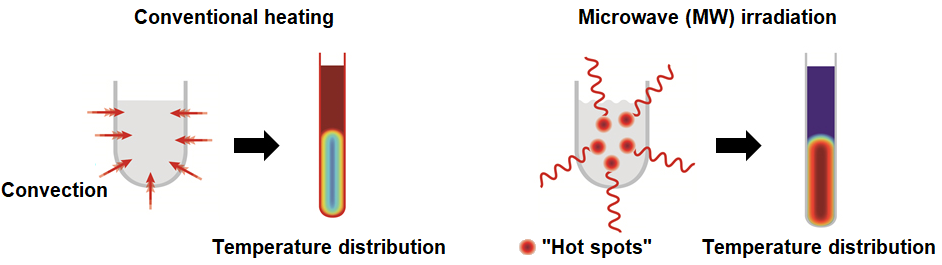
Figure 3. The theoretical background and temperature distribution of conventional and MW heating.
The synthesis of α-hydroxyphosphonic acids was carried out by catalytic hydrogenation of the corresponding dibenzyl esters in a steel autoclave equipped with a pressure gauge (Scheme 3).

Scheme 3. Synthesis of α-hydroxyphosphonic acids by catalytic hydrogenation.
The O-phosphoryloxyphosphonates were synthesized through the reaction of α-hydroxyphosphonates and the corresponding P-chlorides (Scheme 4).
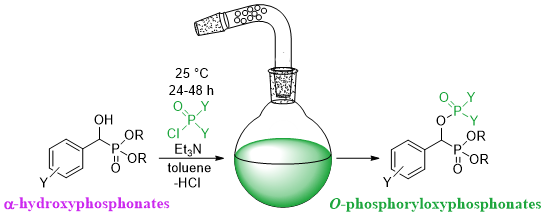
Scheme 4. Synthesis of O-phosphoryloxyphosphonates.
The mechanism of the reactions was studied by computational chemistryR2. The aim of quantum chemical calculations is to determine the energy and the geometry of the starting materials, the product and the transition state which connects them on the potential energy surface. Starting materials and products that are isolable compounds correspond to local energy minima. However, transition states exist only for the duration of a molecular vibration (ca. 10-13 s), thus there are no applicable experimental methods to study their structure. Transition states can be modeled well by quantum chemical calculations (Figure 4). The theoretical methods differ from each other in the approximations they apply while solving the Schrödinger equation for the examined system. Our calculations were performed by Gaussian03 software package using density field theory (DFT), at B3LYP/6-31G(d,p) and B3LYP/6-31++G(d,p) levels of theory.
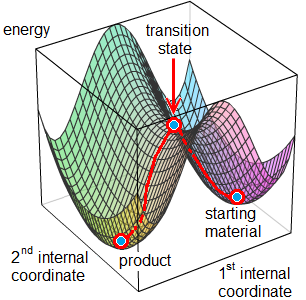
Figure 4. Potential energy surface. The red curve represents the route of the chemical reaction (internal coordinates may be bond lengths or angles).
The cytotoxic assays were carried out in a Hamilton StarLet apparatus equipped with robotic arms. The compounds were tested in a concentration of 200 μM against Mes-Sa uterine sarcoma cell line that produce a fluorescent protein (mCherry) due to genetic modifications. The fluorescence is proportional to the number of cells alive. From this, the proportion of the cells killed by the active substance (hydroxyphosphonate derivative) could be determined.
Results
The research work included the elaboration of a new procedure to synthesize α-hydroxyphosphonates through the reaction of oxo compounds and dialkyl phosphites in the presence of triethylamine as the catalystR1,R3. The main novelty of the new protocol is the minimization of solvent consumption. As a consequence, the environment is polluted by less organic solvents as compared to the previously reported procedures. Due to the “one-pot” reaction and crystallization the products were obtained in high purity (>99%) and in excellent yields (78–95%).
We found that in the absence of triethylamine no product was formed. In the hope of understanding the exact role of triethylamine in the reaction, the mechanism was investigated by theoretical calculations. The calculations pointed out that without triethylamine the activation enthalpy of the reaction is 85.9 kJ/mol, while applying Et3N, it decreased to 68.8 kJ/mol. Triethylamine catalyzes the reaction by facilitating the proton transfer between the phosphite and the oxo compound resulting in a reduced activation gap (Scheme 5)R2.
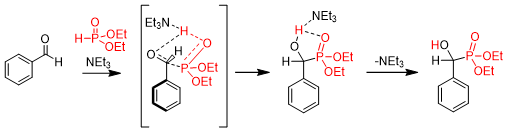
Scheme 5. The calculated mechanism of the triethylamine-catalyzed Pudovik reaction.
According to the single crystal X-ray measurements, hydroxyphosphonate molecules may form either dimers or chain-like associates in the crystal structure. It was concluded that α-hydroxyphosphonate molecules possessing a CH3 function at the α position tend to form dimers. However, hydroxyphosphonates lacking the α-methyl substituent are inclined to form chains (Figure 5).
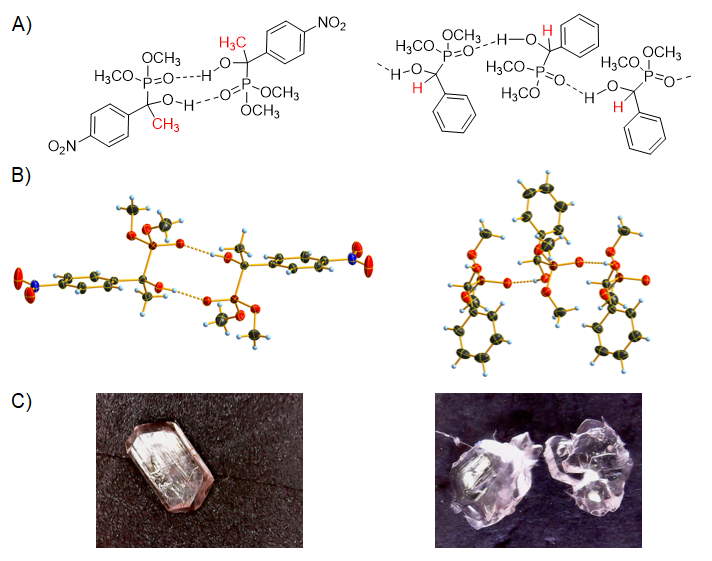
Figure 5. Investigation of the crystal structure of α-hydroxyphosphonates by single crystal X-ray measurements. A) α-Hydroxyphosphonate dimers and chain-like associates. B) X-ray structure of α-hydroxyphosphonates. C) Light microscope images of the single crystals.
To our surprise, the reaction of α-hydroxyphosphonates with primary amines took place within a short reaction time of 15-30 minR2. Encouraged by this interesting finding, we aimed at exploring the mechanism of the reaction by quantum chemical calculations. The enhanced reactivity is attributed to a favorable neighboring group effect that facilitates the reaction (Scheme 6)R2.
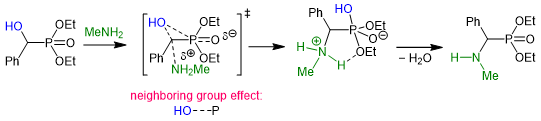
Scheme 6. The formation of α-aminophosphonates is facilitated by a favorable neighboring group effect.
α-Hydroxyphosphonic acids, α-hydroxyphosphonates and O-phosphoryloxyphosphonates were screened against Mes-Sa cells in a concentration of 200 μM. After 48 h of incubation, the fluorescence of the cells was measured and compared to the control experiment containing no hydroxyphosphonate derivative. In Figure 6, zero means that the proliferation of the cells was not inhibited, while 100 means that the measured compound killed all the Mes-Sa cells. Preliminary structure-activity relationships pointed out that aromatic substituents enhanced the activity of the substances.
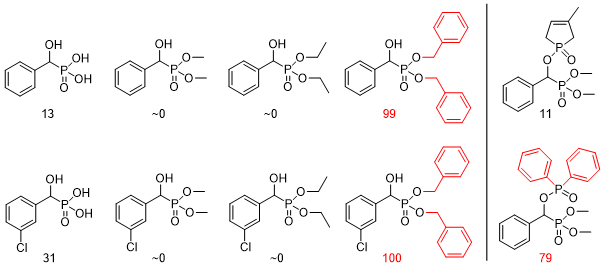
Figure 6. Structure-activity relationships during the cytotoxicity assays of α-hydroxyphosphonic acids, α-hydroxyphosphonates and O-phosphoryloxyphosphonates.
Expected impact and further research
The research work involved the elaboration of a green method to obtain α-hydroxyphosphonates, which was approached from an industrial point of view by minimizing the solvent consumption. The α-hydroxyphosphonates prepared by the new method were subjected to a series of transformations to afford new families of compounds.
The cytotoxicity assays of α-hydroxyphosphonates are a pioneering work, and the preliminary results are indeed promising. To deepen our knowledge on the structure-activity relationships, further derivatives are planned to be synthesized.
Publications, references
Related own articles
Original research articles:
R1. Keglevich, G.; Rádai, Z.; Kiss, N. Z. To date the greenest method for the preparation of α-hydroxyphosphonates from substituted benzaldehydes and dialkyl phosphites. Green Process. Synth. 2017, 6, 197-201. [IF: 0.736]
R2. Kiss, N. Z.; Rádai, Z.; Mucsi, Z.; Keglevich G. Synthesis of α-aminophosphonates from α-hydroxyphosphonates; A theoretical study. Heteroatom Chem. 2016, 27, 260-268. [IF: 1.221]
Short communications:
R3. Rádai, Z.; Kiss, N. Z.; Mucsi, Z.; Keglevich, G. Synthesis of α-hydroxyphosphonates and α-aminophosphonates. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 1564-1565. [IF: 0.809]
R4. Rádai, Z. Zöld módszerekkel a biológiailag aktív anyagokért: Egy értékes vegyületcsalád előállítása. Élet és tudomány, 2018, 10, 303-305. [IF: -]
Book chapters:
R5. Rádai, Z.; Kiss, N. Z.; Keglevich, G. Synthesis of α-hydroxyphosphonates, an important class of bioactive compounds. In: Keglevich György (ed.), Organophosphorus Chemistry: Novel developments. 315 p. Berlin; Boston: Walter de Gruyter, 2018, 91-107. ISBN: 978-3-11-053453-5 [IF: -]
R6. Rádai, Z.; Kiss N. Z.; Keglevich, G. Chalcogenides: Advances in Research and Application (NOVA Science Publishers, New York, USA), 2018, accepted for publication. [IF: -]
Review article:
R7. Rádai, Z.; Keglevich, G. Synthesis and reactions of α-hydroxyphosphonates. Molecules, 2018, 23, 1439. [IF: 3.098 (2017)]
Other publications
Original research articles:
R8. Keglevich, G.; Rádai, Z.; Harsági, N.; Szigetvári, Á.; Kiss, N. Z. A study on the acidic hydrolysis of cyclic phosphinates: 1-Alkoxy-3-phospholene 1-oxides, 1-ethoxy-3-methylphospholane 1-oxide, and 1-ethoxy-3-methyl-1,2,3,4,5,6-hexahydro-phosphinine 1-oxide. Heteroatom Chem. 2017, 28, e21394. [IF: 1.137]
R9. Kiss, N. Z.; Rádai, Z.; Tihanyi, I.; Szabó, T.; Keglevich, G. Microwave-assisted direct esterification of a cyclic phosphinic acid with phenols. Mendeleev Commun. 2018, 28, 31-32. [IF: 2.098 (2017)]
R10. Kiss, N. Z.; Rádai, Z.; Mucsi, Z.; Keglevich, G. The synthesis of bis(phosphinoyl)amines and phosphinoyl–phosphorylamines by the N-phosphinoylation and N-phosphorylation of 1-alkylamino-2,5-dihydro-1H-phosphole 1-oxides. Heteroatom Chem. 2015, 26, 134-141. [IF: 1.203]
Short communications:
R11. Kiss, N. Z.; Rádai, Z.; Keglevich, G. Derivatization of phosphinic acids in the presence of ionic liquids. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 1494-1496. [IF: 0.809]
R12. Kiss, N. Z.; Mucsi, Z.; Rádai, Z.; Böttger, É. V.; Keglevich, G. The synthesis and potential use of cyclic phosphinic acid derivatives. Phosphorus, Sulfur Silicon Relat. Elem. 2015, 190, 668-671. [IF: 0.723]
Review article:
R13. Rádai, Z.; Kiss, N. Z.; Keglevich G. An overview of the applications of ionic liquids as catalysts and additives in organic chemical reactions. Curr. Org. Chem. 2018, 22, 533-556. [IF: 2.193 (2017)]
References
1. Patel, D. V.; Rielly-Gauvin, K.; Ryono, D. E.; Free, C. A.; Rogers, W. L.; Smith, S. A.; DeForrest, J. M.; Oehl, Jr. R. S.; Petrillo, E. W. J. Med. Chem. 1995, 38, 4557-4569.
2. Pokalwar, R. U.; Hangarge, R. V.; Maske, P. V.; Shingare, M. S. Arkivoc, 2006, 11, 196-204.
3. Kategaonkar, A. H.; Pokalwar, R. U.; Sonar, S. S.; Gawali, V. U.; Shingate, B. B.; Shingare, M. S. Eur. J. Med. Chem., 2010, 45, 1128-1132.
4. Song, H.; Mao, H.; Shi, D. Chin. J. Chem., 2010, 28, 2020-2024.
5. Rao, K. U. M.; Sundar, C. S.; Prasad, S. S.; Rani, C. R.; Reddy, C. S. Bull. Korean Chem. Soc. 2011, 32, 3343-3347.
6. Naidu, K. R. M.; Kumar, K. S.; Arulselvan, P.; Reddy, C. B.; Lasekan, O. Arch. Pharm. Chem. Life Sci. 2012, 345, 957-963.
7. Olszewski, T. K. Synthesis, 2014, 46, 403-429.
8. Pudovik, A. N.; Zametaeva, G. A. Izvestiya Akademii Nauk. S.S.S.R., Seriya Khimicheskaya, 1952, 932-939.
9. Nandre, K. P.; Nandre, J. P.; Patil, V. S.; Bhosale, S. V. Chem. Biol. Interface, 2012, 2, 314-321.
10. Kumar, K. S.; Reddy, C. B.; Reddy, M. V. N.; Rani, C. R.; Reddy, C. S. Org. Commun., 2012, 5, 50-57.
11. Aouani, I.; Lahbib, K.; Touil, S. Medicinal Chem., 2015, 11, 206-213.
12. Ramananarivo, H. R.; Solhy, A.; Sebti, J.; Smahi, A.; Zahouily, M.; Clark, J.; Sebti, S. ACS Sustain. Chem. Eng., 2013, 1, 403-409.
13. Kalla, R. M. N.; Lee, H. R.; Cao, J.; Yoo, J. W.; Kim, I. New J. Chem., 2015, 39, 3916-3922.
14. Keglevich, G.; Kiss, N. Zs.; Mucsi, Z.; Körtvélyesi, T. Org. Biomol. Chem. 2012, 10, 2011-2018.
