|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Dr. Keglevich György
The Deoxygenation of Phosphine Oxides: Environmentally Friendly Approaches
Introducing the research area
The use of environmentally friendly and inexpensive procedures is very important in chemical industry, too. During the synthesis planning, it is advisable to keep in mind the principles of green chemistry. For this reason, the harmful and dangerous reagents should be avoided, minimizing the use of excipients and the formation of byproducts. During my research, I am studying the deoxygenation of various tertiary phosphine oxides to develop environmentally friendly solutions. I plan to develop a reduction method that would allow the synthesis of tertiary phosphines more economically and efficiently (Fig. 1).
Fig. 1: The main aspects of deoxygenation
Brief introduction of the research place
Our research group operating at the Department of Organic Chemistry and Technology is led by prof. György Keglevich, and engaged with environmental-friendly and organophosphorus chemistry, has a history of several decades, performing important industrial streamlining reactions, producing organophosphorus compounds of pharmaceutical significance, synthesizing and testing new catalysts and conducting reactions in microwave reactors.
History and context of the research
The deoxygenation of tertiary phosphine oxides is an important reaction, as it provides useful intermediates and reagents. Tertiary phosphines are widely used as ligands in transition metal complexes and as starting materials and reagents in syntheses [1]. The transition metal (most frequently Pd, Pt or Rh) complexes may be applied in homogeneous catalytic hydrogenations, hydroformylations, hydrosilylations or palladium-catalyzed coupling reactions [K1, K2]. Tertiary phosphines may be the starting materials for quaternary phosphonium salts used as surfactants, ionic liquids or phase transfer catalysts. Representative examples for the application of tertiary phosphines as reagents in various organic reactions include the Wittig reaction (formation of C=C bonds), the Mitsunobu reaction (formation of C–O or C–N bonds), and Appel-type reactions (formation of C–halogen bonds).
Unfortunately, the mentioned reactions are of poor atom economy, leading to substantial amounts of phosphine oxide waste. The phosphine oxides formed are undesirable by-products, and should be regenerated and recycled (Fig. 2) [2]. The simplest method to regenerate phosphines from phosphine oxides is reduction [K3].
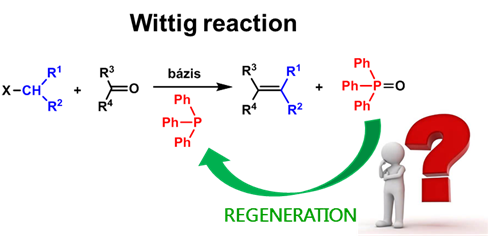
Fig. 2: Regeneration of phosphine through the Wittig reaction example
The research goal, open questions
The most general method for the deoxygenation of phosphine oxides to phosphines involves the application of silanes. A wide spectrum of silanes has been described as P=O deoxygenating agents. These days, it is a real challenge to accomplish the deoxygenation of phosphine oxides by cheap and user-friendly silanes, elaborating green chemical protocols (Fig. 3) [3, K4].
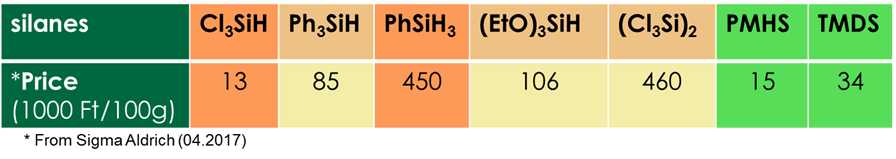
Fig. 3: Some commercially available silane reducing agents
Within silanes, trichlorosilane (Cl3SiH) is the most widely used reagent, but it is volatile (bp: 32 °C) and corrosive, implying a significant disadvantage. Phenylsilane (PhSiH3) may be a good choice as the reagent for the deoxygenation; however, it is rather expensive. The group of user-friendly silanes include 1,1,3,3-tetramethyldisiloxane (TMDS) and polymethylhydrosiloxane (PMHS). These derivatives were used satisfactorily to give phosphines, although they were not as reactive as e.g. PhSiH3 or Cl3SiH. Hydrosiloxanes with Si–O–Si moieties are more stable and the ultimate waste is silica. To promote their reactivity, the deoxygenations by TMDS and PMHS were performed with copper-, titanium- and indium-catalysis, or with a phosphoric acid diester as the catalyst [4,5]. However, the justification of the catalyst is sometimes questionable. Deoxygenation methods in the literature are generally not considered to be simple, inexpensive, effective, environmental-friendly reactions. Therefore, we are trying to develop an easy-to-implement and environmental-friendly synthesis for deoxygenation of phosphine oxides.
In the literature, the microwave (MW) heating has proved its efficiency in several different syntheses [6, K5, K6]. However, this technique has not been used previously in similar deoxygenation processes.
Methodology
During my research, the reduction transformations were carried out using two heating methods: conventional heating (in a tempered oil bath) or MW reactor. In both cases, the reactions were carried out under pressure (in closed vessels), using a glass bomb (a thick-wall glass tube that can be closed) or a commercial MW vial with the same geometry (Fig. 4). The MW-assisted reactions were carried out in a CEM Discover [300 W] microwave reactor equipped with a pressure controller (Fig. 4).

Fig. 4: The reaction vessels used for deoxygenation (left) CEM MW reactor (right)
Traditionally, syntheses have been achieved through conductive heating
which is a slow and inefficient process, where heat is driven into the
reaction mixture, passing first through the walls of the vessel. Nevertheless,
microwaves interacts directly
with the molecules resulting in an instantaneous localized so-called
“superheating”. In the case of MW irradiation, polar molecules try to align
themselves with the changing electric field of MW – this interaction is called
dipole rotation – leading to a rapid rise in temperature (Fig. 5) [7].
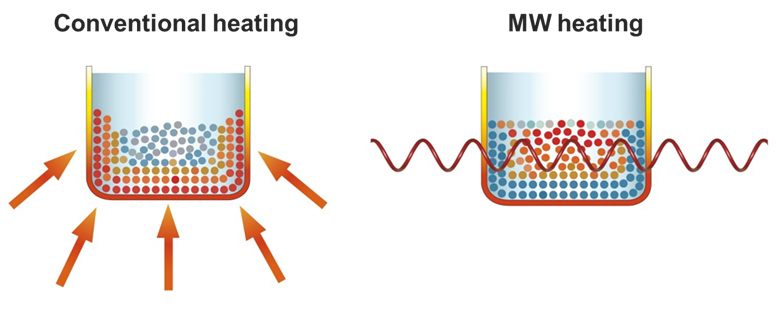
Fig. 5: Applied heating modes
Results
Deoxygenation of phosphine oxides was carried out in a solvent or under solvent-free conditions and by conventional heating or microwave irradiation (MW). It was found that without any solvent the reductions were more rapid. With respect to the heating mode we have experienced the favorable (reaction-accelerating) effect of MW. So the most efficient transformations were achieved by this method (the reaction time required for complete conversion was the smallest in this case) (Fig. 6) [K7, K8].
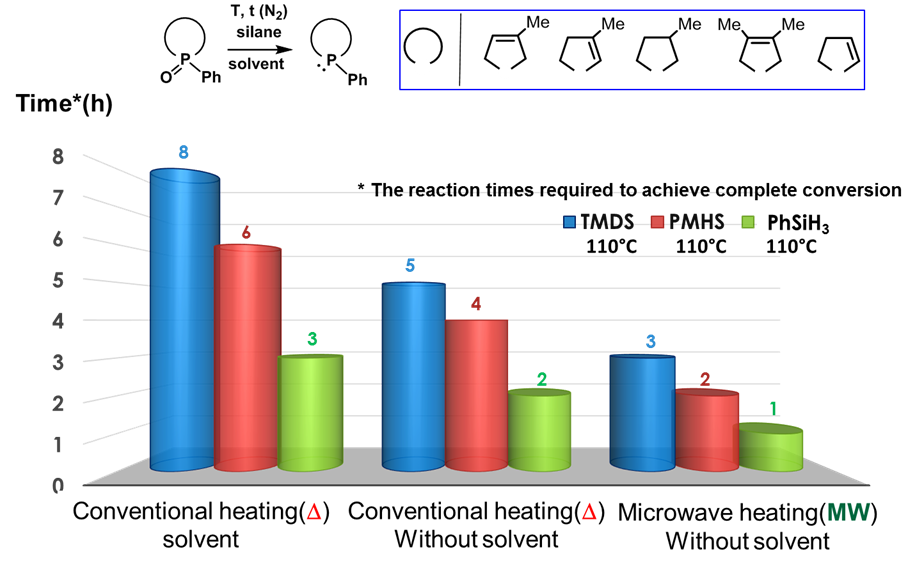
Fig. 6: Deoxygenation of phosphine oxides under various conditions
TMDS and PMHS were less effective than phenylsilane (PhSiH3), but the efficiency of transformation in solvent-free MW transformations increased considerably. This means that these cheaper silicone industrial by-products can also be used effectively and environmentally friendly for deoxygenation without using catalysts (Fig. 7).
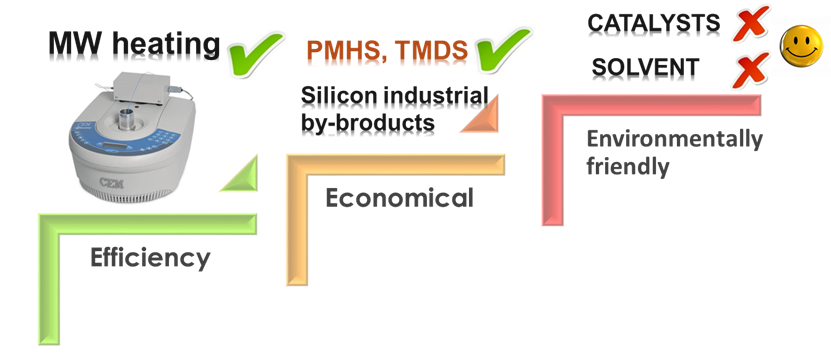
Fig. 7: Characteristics of the developed deoxygenation method
During the experiments, we tested a variety of silane reducing agents
(produced in connection with a Graz cooperation). Those silanes were found
most effective where three hydrogen atoms attached to the silicon atom. With
the increase in the size of the other substituents associated with the silicon
atom, the reactivity of the silanes was generally lower [K9, K10].
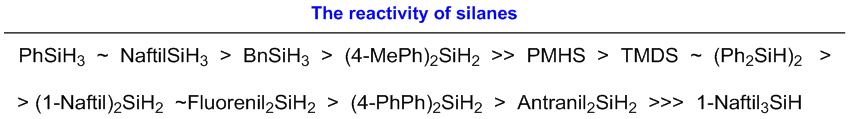
Expected impact and further research
The environmental-friendly method developed for the deoxygenation of a specific family of compounds is of a more general value and thus other phosphine oxides can be deoxygenated under similar conditions. Using the efficient deoxygenation method (which was carried out with phenylsilane under MW and solvent-free conditions) developed by us, our group successfully implemented the deoxygenation of bis(phosphonomethyl)amines to obtain valuable ligands [8]. TMDS and PMHS turned out to be generally usable deoxygenation agents under MW and solvent-free conditions offering a greener chemical method. The results of the deoxygenation of phosphine oxides and fragmentation of the bridged phosphine oxides contribute to the expansion of MW technology.
Publications, references, links
Publications:
[K1] Bagi, P.; Kovács, T.; Szilvási, T.; Pongrácz, P.; Kollár, L.; Drahos, L.; Fogassy, E.; Keglevich, G. J. Organomet. Chem. 2014, 751, pp. 306–313
[K2] Bagi, P.; Kovács, T.; Kollár, L.; Fogassy, E.; Keglevich, G. Phosphorus, Sulfur, Silicon 2015, 190 (5-6), pp. 821–823
[K3] Kovács, T. and Keglevich, G. Curr. Org. Chem. 2017, 21, pp. 569-585
[K4] Kovács, T. and Keglevich, G. Phosphorus, Sulfur, Silicon 2016, 191, pp. 359-366
[K5] Keglevich, G.; Kiss, N. Zs.; Bálint, E.; Bagi, P.; Grün, A.; Kovács, T.; Henyecz, R. and Ábrányi-Balogh, P. Phosphorus, Sulfur, Silicon 2016, 191, pp. 1416-1420
[K6] Keglevich, G.; Kiss, N. Zs.; Grün, A.; Bálint, E., Kovács, T. Synthesis, 2017, in press.
[K7] Keglevich, G.; Kovács, T. Curr. Green Chem. 2014, 1, pp. 182–188
[K8] Keglevich, G.; Kovács, T.; Csatlós, F. Heteroatom Chem. 2015, 26, pp. 199–205
[K9] Kovács, T.; Urbanics, A.; Csatlós, F.; Binder, J.; Falk, A.; Uhlig, F.; Keglevich, G. Current Org. Synth. 2016, 13(1), pp. 148–153
[K10]Kovács, T.; Urbanics, A.; Csatlós, F.; Keglevich, G.
2017, in press (DOI: 10.1002/hc.21376).
Links:
References:
[1] Hérault, D.; Nguyen, D. H.; Nuel, D.; Buono, G. Chem. Soc. Rev. 2015, 44, 2508
[2] Van Kalkeren, H. A.; Blom, A. L.; Rutjesa, F. P. J. T.; Huijbregts, M. A. J. Green Chem. 2013, 15, 1255
[3] Quin, L. D. A guide to organophosphorus chemistry; John Wiley & Sons: New York, 2000
[4] Li, Y. H.; Lu, L. Q.; Das, S.; Pisiewicz, S.; Junge, K.; Beller, M. J. Am. Chem. Soc. 2012, 134, 18325
[5] Li, Y. H.; Das, S.; Zhou, S. L.; Junge, K.; Beller, M. J. Am. Chem. Soc. 2012, 134, 9727
[6] Keglevich, G. Magy. Kém. Folyóirat 2008, 114, 81
[7] Hayes, L. B.; Microwave Synthesis Chemistry at the Speed of Light, CEM Publishing, USA, 2002, pp. 11-25
[8] Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. J. Org. Chem. 2016, 81, pp. 111-121
