|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Dr. Nagy Zsombor Kristóf
Investigation and Downstream Processing of Electrospun Amorphous Solid Dispersion
Introducing the research area
In the last few decades more and more poorly water-soluble drugs with low bioavailability appeared in the drug discovery pipelines. To tackle this issue, amorphous solid dispersions have been introduced. These materials, composites can be gently and cost-effectively manufactured by electrostatic spinning. My research work is centered on the upscaling of the technology, the characterization and the downstream processing of the obtained nanofibrous dispersions.
Brief introduction of the research place
My research work is conducted at the Department of Organic Chemistry and Technology of Budapest University of Technology and Economics with the supervision of Dr Zsombor Kristóf Nagy and Dr György Marosi. They own more than 10 years of experience in this area and are co-authors of many relevant publications and some patents.
History and context of the research
From the 1980’s, the pharmaceutical industry has begun applying high-throughput screening and computational modelling to discover original, new chemical entities. These methods can find molecules with good permeability (through phospholipid membranes) and high activity to the receptor, but weak solubility as hits. As a result, active drugs have been discovered in the last decades. However, it is not sure if they may constitute a product that can be brought to market, since their low aqueous solubility can impede their absorption and thus mitigates their effectiveness. Therefore, one of the largest challenges in pharmaceutical industry is to formulate these newly discovered drugs. Reformulating previously discovered drugs to enhance the originally poor bioavailability is also of great interest.
Amorphous solid dispersions were created to tackle this issue [1, 2]. Under this term we mean the molecularly dispersed dispersion of an active pharmaceutical ingredient in a polymer matrix. The drug becomes amorphous from crystalline during the preparation, whereby it will show an enhanced dissolution. The manufacturing of such dispersion can be carried out by electrospinning that might be able to eliminate drawbacks related to the two most commonly applied technologies, i.e. melt extrusion and spray drying. With electrospun nanofibers, their huge surface area also increases the dissolution rate according to the Noyes-Whitney equation [3, 4]. However, despite their obvious advantages, electrospinning and electrospun materials have not been not applied in the pharmaceutical industry as yet.
The research goal, open questions
The research was/is conducted in collaboration with foreign and Hungarian pharmaceutical companies. Based on this, we would like to answer if electrospinning and the so-obtained solid dispersions can play a significant role in the pharmaceutical industry. It can be considered an important question if high speed electrospinning developed by our research group can achieve the required level of productivity. Another very important aspect is the physical stability of the produced fibers. Namely, amorphous materials are prone to convert back to the thermodynamically stable crystalline counterpart. Therefore, long-term stability of the electrospun dispersions must be investigated both in pure and tablet forms.
According to literature, there had been no publications on the downstream processing and tableting of electrospun materials prior to commencing our work, although this step is inevitable for industrial applications. Our goal was to prepare conventional tablets with fibers, and to study the compatibility of the electrospun amorphous drug with commonly applied excipients.
Methods
High speed electrospinning: The solution (formed with organic solvents or water) of the drug and the polymer is fed by a peristaltic pump to a rotating, stainless steel spinneret attached to high voltage. Fibers jet from the edge of the spinneret and get elongated, usually to the nano range in diameter, due to the electrostatic and centrifugal forces. The solvent is evaporated really quickly, thus the time is too short for the crystallization of the drug. The fibers can then be collected on a grounded collector or by special collection systems (e.g. knife removal).
Characterization of the electrospun solid dispersions and the tablets was performed by the following analytical techniques: scanning electron microscopy, differential scanning calorimetry, X-ray powder diffraction, dynamic vapour sorption, solid state NMR spectroscopy, in vitro dissolution testing, chemical mapping based on Raman spectrometry, hardness testing, disintegration time measurement, near infra-red and Raman spectrometry.
Results
Comparing electrospinning with the industrially applied solvent-based technology, spray drying, it can be stated that much more concentrated solutions can be manufactured with fiber spinning technology. Hence, less solvent is evaporated while producing the same amount of dry dispersion, which is essential to comply with pharmaceutical regulations. Additionally, solid dispersion with a much larger surface area (nanofibers) can be obtained, which means faster dissolution (Fig. 1). Furthermore, we can observe no significant difference between dissolution of fibers manufactured by the two different technologies (single-needle, small-scale and high speed electrospinning). The productivity achieved was 450 g/h, which seems fairly satisfying. This value can even be increased by varying the size and number of spinnerets. The dissolution rate and extent is much higher than in the case of the crystalline drug (or its physical mixture with the polymer).
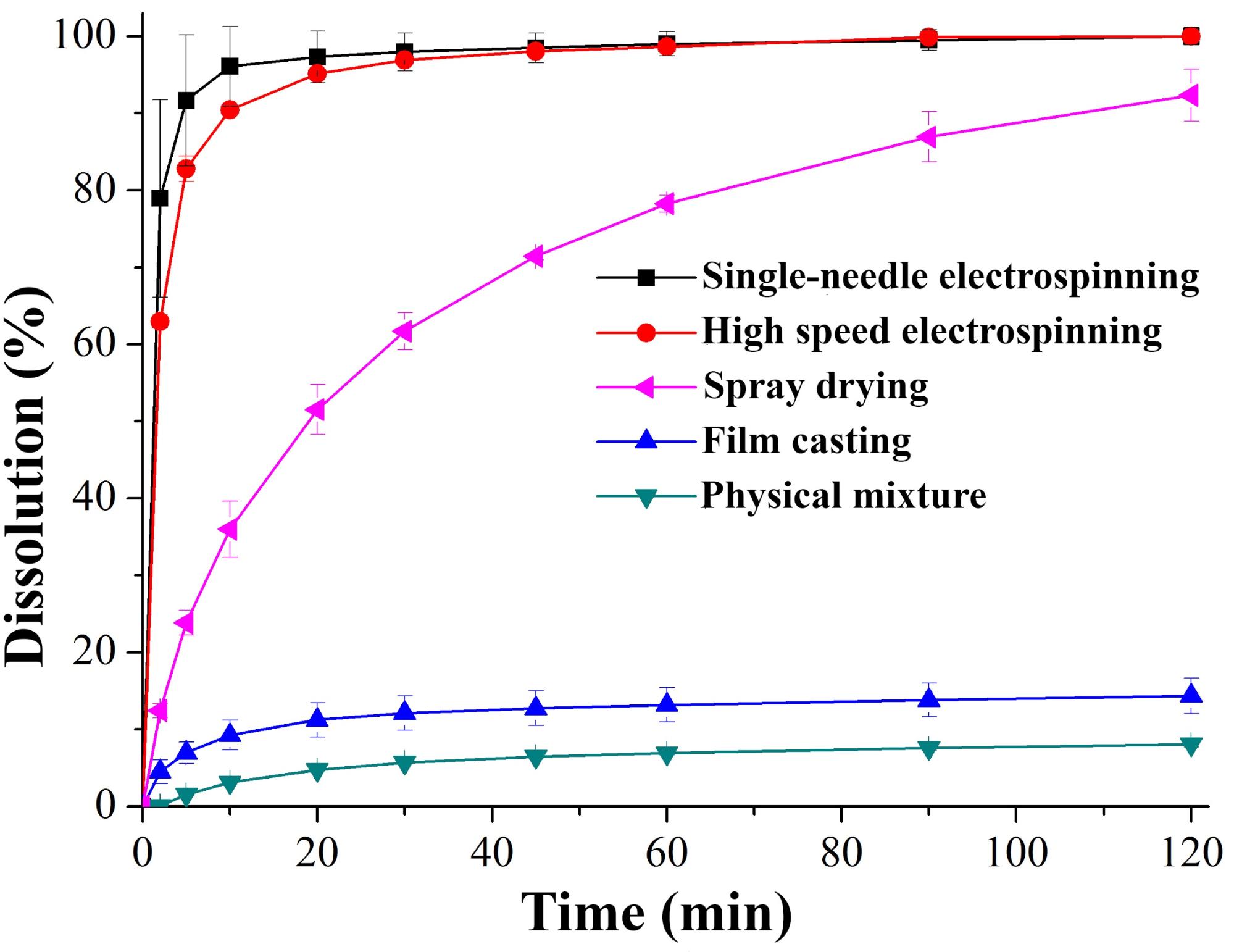
Fig. 1: Dissolution of itraconazole from differently manufactured solid dispersions with vinylpyrrolidone-vinyl acetate copolymer (PVPA64)
The different nanofibrous samples kept in climate chambers for a year released itraconazole in similar way than after production (controlled conditions, PVPVA64: 25 °C/60% relative humidity, hydroxypropyl methylcellulose (HPMC): 40 °C/75% relative humidity) (Fig. 2).
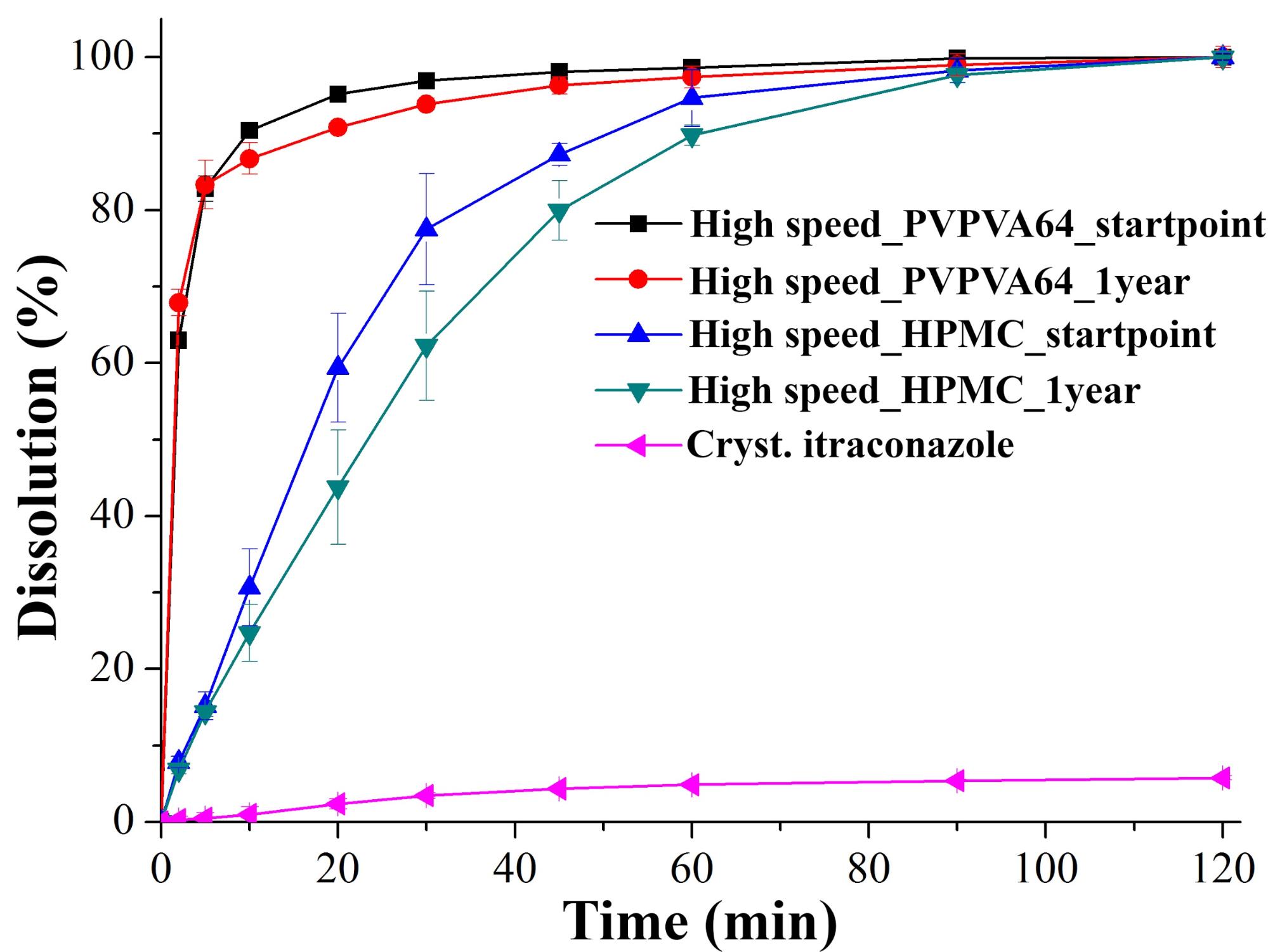
Fig. 2: Dissolution of itraconazole from solid dispersions manufactured by high speed electrospinning, after production and after one year of storage
In the next stage of the work, we were dealing with the downstream processing, conversion to tablets of solid dispersion comprising itraconazole and PVPVA64. The fibers were ground by oscillatory milling and compressed into tablets after conventional excipients. According to the latest pharmaceutical industry trends, an optimizing design of experiments was applied to determine the appropriate compression force and composition. The downstream processing and the excipients used (mannitol, microcrystalline cellulose, silicon dioxide, crospovidone and sodium stearyl fumarate) did not cause any physical instability as it was proved by the unchanged dissolution profile (Fig. 3). Furthermore, chemical degradation did not occur either, since the applied chromatography method showed 0.08% more impurity in tablets stored for a year than in the crystalline material.
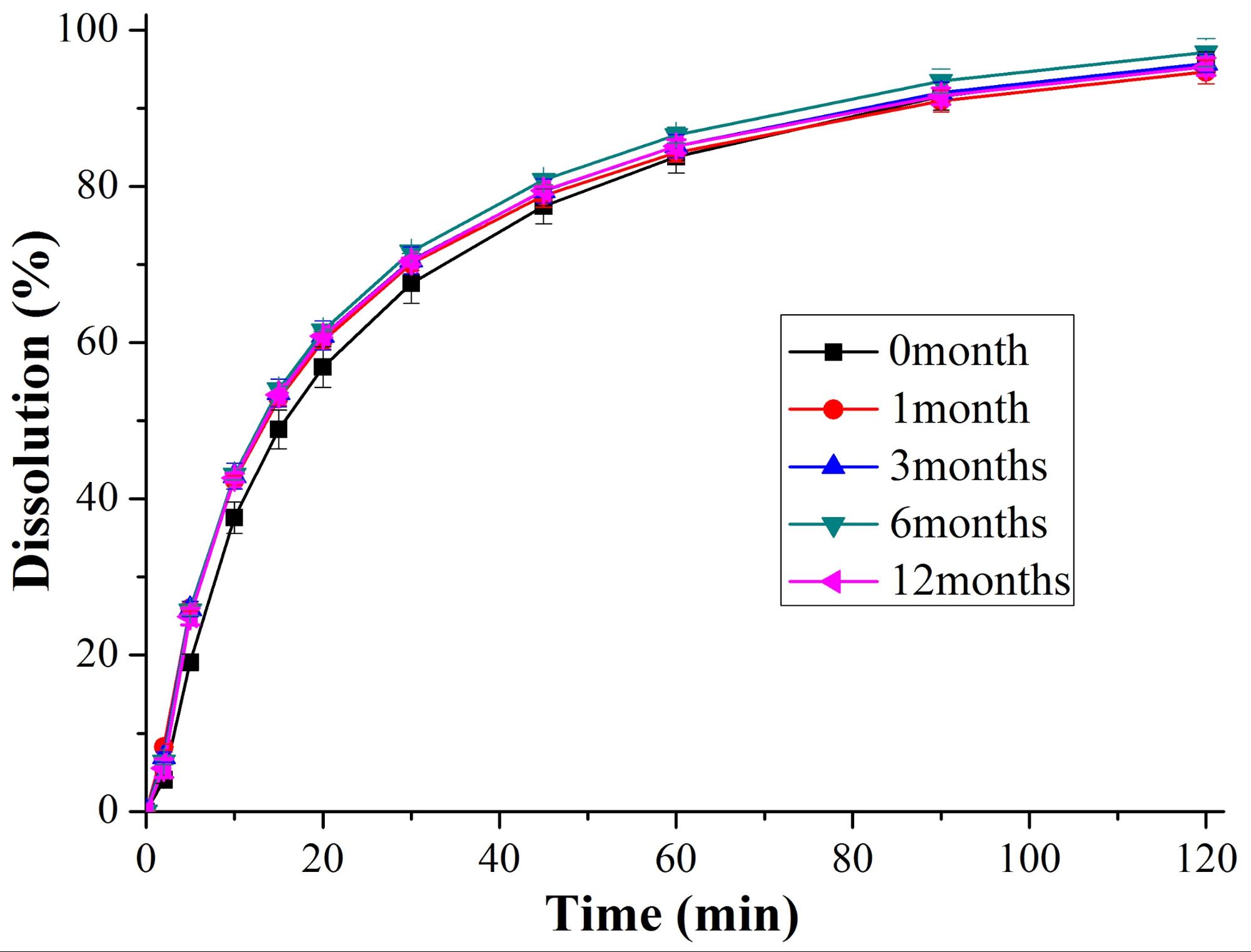
Fig. 3: Dissolution of itraconazole from solid dispersion containing tablets stored at controlled conditions (25 °C/60% relative humidity)
Film coating with aqueous suspensions can be deemed as a critical step of the downstream processing in the case of amorphous solid dispersions. Water increases the mobility of the drug in the dispersion whereby it facilitates its crystallization. However, our film-coated tablets released the incorporated drug in the same way as uncoated cores.
One of the most exciting part of the research is the investigation of the (in)compatibility of the amorphous drug (in our case, itraconazole) and common excipients. Magnesium stearate is the most used lubricant though its hydrophobicity was proved to deteriorate dissolution of certain drugs. During our research we observed a previously undescribed phenomenon caused by magnesium stearate. Stearic acid deriving from magnesium stearate in the acidic dissolution medium formed a secondary bond (hydrogen bond) with the triazole moiety of itraconazole (based on solid state NMR spectroscopy and Raman spectrometry). Both parts of the associate formed in this way have low aqueous solubility, thus lower dissolution extent was noticed than with pure amorphous itraconazole. This issue can be avoided by the application of a different lubricant (e.g. sodium stearyl fumarate) or by using HPMC in the original solid dispersion as matrix. This polymer stabilizes itraconazole through hydrogen bonds.
The generalizability of downstream processing of electrospun nanofibers was proved by the conversion of another fibrous solid dispersion containing flubendazole (matrix: hydroxypropyl-ß-cyclodextrin and polyvynilpyrrolidone) into tablets by direct compression. The determination of drug contents in these tablets by non-destructive methods can be considered as a pioneering research. Near infra-red spectrometry was found slightly superior (to Raman), nevertheless both techniques can be suitable for drug content determination and for the investigation of content uniformity in solid dispersion-based tablets.
Expected impact and further research
Subjects directly related to the presented work have been published in 6 publications. Of this six, five belong to the top 25% (Q1), three belong to the top 10% (D1) in this discipline. Furthermore, four other publications have also been accepted, which are indirectly related to the work. Due to the novelty of the research and its industrial importance, similar interest and acceptance can be expected from the journals.
Continuation of the work will be performed in accordance with the pharmaceutical trends. The largest foreseen change in the industry is the shift from batch to continuous manufacturing. Accordingly, we would like to study the continuous wet and melt granulation and dry granulation of electrospun and other amorphous solid dispersions. Furthermore, we would like to develop real-time analytical techniques to support these technologies.
Publications, references, links
Directly related publications
[I]: Z. K. Nagy, A. Balogh, B. Démuth, H. Pataki, T. Vigh, B. Szabó, K. Molnár, B. T. Schmidt, P. Horák, G. Verreck, I. Van Assche, M. E. Brewster, Int. J. Pharm., 2015, 480 (1-2), pp. 137‒142
IF: 3,994; Independent citations: 20; Dependent citations: 13
[II]: B. Démuth, Z. K. Nagy, A. Balogh, T. Vigh, G. Marosi, G. Verreck, I. Van Assche, M. E. Brewster, Int. J. Pharm., 2015, 486 (1‒2), pp. 268-286
IF: 3,994; IC: 23; DI: 3
[III]: B. Démuth, A. Farkas, H. Pataki, A. Balogh, B. Szabó, E. Borbás, P. L. Sóti, T. Vigh, É. Kiserdei, B. Farkas, J. Mensch, G. Verreck, I. Van Assche, G. Marosi, Z. K. Nagy, Int. J. Pharm., 2016, 498 (1‒2), pp. 234-244
IF: 3,649; IC: 5; DC: 2
[IV]: B. Démuth, A. Farkas, A. Balogh, K. Bartosiewicz, B. Kállai-Szabó, J. Bertels, T. Vigh, J. Mensch, G. Verreck, I. Van Assche, G. Marosi, Z. K. Nagy, J. Pharm. Sci., 2016, 105 (9), pp. 2982‒2988
IF: 2,713; IC: 3; DC: 1
[V]: T. Vigh, B. Démuth, A. Balogh, D. L. Galata, I. Van Assche, C. Mackie, M. Vialpando, B. Van Hove, P. Psathas, E. Borbás, H. Pataki, P. Boeykens, G. Marosi, G. Verreck, Z. K. Nagy, Drug Dev. Ind. Pharm., 2017, 43 (7), pp. 1126‒1133
IF: 2,295, IC: 1; DC: 0
[VI]: B. Démuth, A. Farkas, B. Szabó, A. Balogh, B. Nagy, E. Vágó, T. Vigh, A. P. Tinke, Z. Kazsu, Á. Demeter, J. Bertels, J. Mensch, A. Van Dijck, G. Verreck, I. Van Assche, G. Marosi, Z. K. Nagy, Adv. Powder Technol., 2017, 28 (6), pp. 1554‒1563
IF: 2,659; IC: 0; DC: 0
Indirectly related publications
[VII]: A. Balogh, R. Cselkó, B. Démuth, G. Verreck, J. Mensch, G. Marosi, Z. K. Nagy, Int. J. Pharm., 2015, 495 (1), pp. 75‒80
IF: 3,994; IC: 5; DC: 1
[VIII]: E. Borbás, B. Sinkó, O. Tsinman, K. Tsinman, É. Kiserdei, B. Démuth, A. Balogh, B. Bodák, A. Domokos, G. Dargó, G. T. Balogh, Z. K. Nagy, Mol. Pharm., 2016, 13 (11), pp. 3816‒3826
IF: 4,440; IC: 0; DC: 0
[IX]: A. Farkas, B. Nagy, B. Démuth, A. Balogh, H. Pataki, Z. K. Nagy, G. Marosi, J. Chemom., 2017, 31 (1), e2861, pp. 1-11
IF: 1,884; IC: 0; DC: 0
[X]: A. Balogh, B. Farkas, Á. Pálvölgyi, A. Domokos, B. Démuth, G. Marosi, Z. K. Nagy, J. Pharm. Sci., 2017, 106 (6), pp. 1634‒1643
IF: 2,713; IC: 0; DC: 0
References
[1]: K. Sekiguchi, N. Obi, Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man, Chem. Pharm. Bull., 1961, 9 (11), pp. 866‒872
[2]: W. L. Chiou, S. Riegelman, Pharmaceutical applications of solid dispersion systems, Journal of Pharmaceutical Sciences, 1971, 60 (9), pp. 1281‒1302
[3]: A.A. Noyes, W.R. Whitney, Drug Dissolution, J. Am. Chem. Soc., 1897, 19 (12), pp. 930‒934
[4]: Z.K. Nagy, A. Balogh, B. Vajna, A. Farkas, G. Patyi, Á. Kramarics, G. Marosi, Comparison of electrospun and extruded soluplus®-based solid dosage forms of improved dissolution, J. Pharm. Sci., 2012, 101 (1), pp. 322‒332
