 |
BMe Research Grant |

|
Oláh György Doctoral School, BME
Department of Organic Chemistry and Technology
Supervisor: Prof. Péter Huszthy
Synthesis and studies of sensor and selector molecules based on acridino-crown ethers
Introducing the research area
Crown ethers offer unusual
opportunities for the study of molecular
recognition. These kinds of studies are important not only because they help better understand molecular recognition in living organisms, but they may also lead to the development of effective sensor
and selector molecules.
Brief introduction of the research place
In Hungary, research started with crown ethers containing N-heterocyclic subunit in the Organic Chemistry Group of the Department of Organic Chemistry and Technology by Prof. Péter Huszthy. Our research group deals with the synthesis, molecular recognition studies (metal ion selectivity and enantioselectivity) and applications of these kinds of achiral and optically active macrocycles.
History and context of the research
Selective complexation properties of crown
ether-type sensor and selector molecules are based on molecular recognition,
when the host molecule selectively binds a certain  type of guest
molecule through noncovalent bonding
(Fig. 1). Molecular recognition is an ubiquitous in Nature.
Examples include the double helix of DNA, or the specificity of enzymes and receptors.
Even up to a few decades ago molecular recognition was considered a pure biological
phenomenon. However, recent successes of supramolecular
chemistry have demonstrated that biological behaviour can be imitated using
small molecules. [1, 2]
type of guest
molecule through noncovalent bonding
(Fig. 1). Molecular recognition is an ubiquitous in Nature.
Examples include the double helix of DNA, or the specificity of enzymes and receptors.
Even up to a few decades ago molecular recognition was considered a pure biological
phenomenon. However, recent successes of supramolecular
chemistry have demonstrated that biological behaviour can be imitated using
small molecules. [1, 2]
The basic principles of crown ether chemistry was established by the pioneering work of Pedersen, who observed the unique complexation properties of a cyclic polyether (dibenzo-18-crown-6 ether) toward alkali and alkaline earth metal ions. He recognized that depending on their sizes, crown ethers can form complexes of different stability with chemically similar metal ions. [3]
Figure 1. Molecular recognition
Crown ethers containing a pyridine unit show outstanding complexation properties towards heavy metal ions and protonated primary amines, because of the soft nitrogen atom and the aromatic ring. [4, 5, 6] Studies on the complexation of the optically active pyridino-crown ethers and the enantiomers of chiral protonated primary aralkyl amines have proved that the enantioselectivity is based on three independent interactions: tripodal hydrogen bonding between the pyridine nitrogen and two alternating oxygen of the macroring and the protons of the ammonium salt, π–π stacking between the aromatic moieties of the host and the guest, and steric repulsion between the substituents on the chiral centers of the crown ethers and certain aromatic protons of the ammonium salts.
The aromatic π -system of acridino-macrocycles, which is more extended compared to pyridino-crown
ethers, not only
enhances π–π
interaction, but also makes the structure of the macroring more rigid. Both
factors improve selectivity. Furthermore, they are also fluoro- and chromogenic,
so their complexation can be studied by sensitive photophysical methods, such as
fluorescence spectroscopy.
The research goal, open questions
Development of sensor and selector molecules
capable of selective recognition of different metal ions, organic ionic and
neutral species or the enantiomers of chiral
molecules is of great importance for many applications in medical chemistry,
environment protection, pharmaceutical, food, cosmetic and pesticide
industries.
Our aim was to synthesize new acridino-18-crown-6
ether type achiral and enantiomerically pure sensor and selector molecules. The
complexation properties of these ligands toward different metal ions and
enantiomers of protonated primary organic amines were studied using
potentiometric, fluorescence spectroscopic and other methods. [7, 8, 9, 10]
Methodology
Synthesis of the compounds was carried out using modern methods of preparative organic chemistry. Purity of the compounds was determined by thin layer chromatography, measuring melting points and optical rotations. Structure of the compounds was determined using IR, 1H- and 13C-NMR, MS and EPR spectroscopies and elemental analysis.
The metal ion selectivity and enantioselectivity of the new ligands were studied using potentiometric, fluorescence spectroscopic, stirring and chromatographic methods.
Potentiometry
is an electroanalytical method, where determination of the measured component
is based on the electrode potential of the working electrode (for example,
ionselective electrode containing an ionophore incorporated into a PVC membrane)
immersed into the measured solution. As the electrode potential can’t be
measured directly, the electromotoric force of an electrochemical cell
containing the working electrode, the measured solution and a reference
electrode having a constant potential is measured instead. [11, 12]
In the case of optical sensors, determination of the chemical interaction between the sensor and a guest molecule is accomplished measuring optical phenomena, for example fluorescence. [13, 14] Fluorescence is a phenomenon, where an electron of a molecule relaxes to its ground state emitting light (usually of longer wavelength, and therefore lower energy, than the absorbed radiation) after excited by absorbing UV or visible light. When taking a fluorescence spectrum, the solution of an analyte is irradiated using light of a single wavelength, and the intensity of the emitted light is measured as a function of wavelength.
Immobilization of crown ethers on a solid support
combines the selectivity of the macrocycle with the recyclability and the
reusability of the stationary phase. Synthesis of ligands is expensive and
difficult, so to avoid material loss, they have to be immobilized, for example, on silica gel. Complexation
properties of immobilized macrocycles can be studied using
stirring method (where the immobilized ligand is stirred in a
solution of guest molecules until the equilibrium is reached, when the degree of
complexation is measured), or using chromatographic
method (where guest molecules are separated using a column filled with the
stationary phase).
Results
My PhD work aimed at the synthesis of crown ethers containing an acridino
heterocyclic unit and studying their
molecular recognition capability. In my PhD dissertation I described the synthesis of twenty
one new compounds – seven enantomerically pure and fourteen achiral ones – and
four known compounds by a new method. [7, 8, 9, 10]
We synthesized the enantiomerically pure (R,R)-1–(S,S)-4 crown ethers (Fig. 2) to study their enantioselective complexation properties with protonated primary amines and protonated amino-acid esters and also their metal ion selectivity.
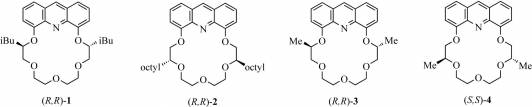
Figure 2. Enantiomerically pure acridino-18-crown-6 ethers
Enantio- and metal ion selectivities of
acridino-crown ethers (R,R)-1 containing isobutyl groups closer to
the aromatic ring, and (R,R)-2 containing octyl groups further
away from the aromatic moiety were studied using potentiometry. We found that
the positions of the substituents on the chiral centers significantly affect
enantioselectivity: ligand (R,R)-1 showed high entantioselectivity
toward the enantiomers of 1-phenylethylammonium ion (ΔlogK=0.25), while ligand
(R,R)-2 showed poor enantioselectivity. The latter showed high
selectivity toward Ag(I) even in the presence of Hg(II) and Cu(II) (ΔlogK≥4.5),
which usually cause high interference. [7]
The enantiomeric recognition ability of
diisobutyl-substituted (R,R)-1, dimethyl-substituted
(R,R)-3 and dimethyl substituted (S,S)-4
acridino-crown ethers was studied toward the enantiomers of 1-phenylethylamine,
1-(naphth-1-yl)ethylamine, phenylglycine methyl ester and phenylalanine methyl
ester hydrogen perchlorate salts using fluorescence spectroscopy.
In
accordance with our previous potentiometric results, we found that the bulkiness
and the position of the alkyl groups on the chiral centers have significant
effect on the degree of enantioselectivity. Among the studied salts, ligand
(R,R)-3 showed greatest enantioselectivity, toward the enantiomers
of 1-(naphth-1-yl)ethylammonium ion (ΔlogK=0.41). [8]
By alkylating the secondary amino group of
macrocycles 5 and 6 we synthesized new sensor (7–10)
and selector (SP-11) molecules (Fig. 3).
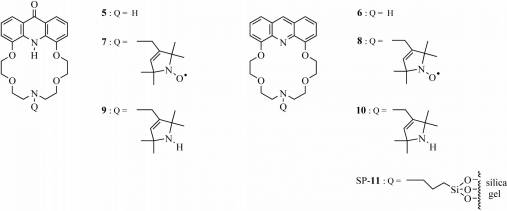
Figure 3. Macrocycles
containing a nitrogen atom in their macroring
As the mechanism of intramolecular fluorescence quenching is
unclear for double labeled compounds
(which contain a fluorophore and also a nitroxyl radical so they can be studied
using fluorescence or also EPR
spectroscopy), the synthesis and 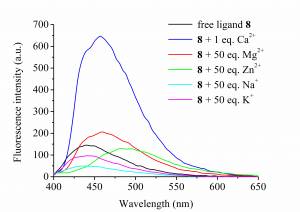 studies of
these double sensors are of great importance. [15] Double sensors containing a crown ether part have not been
reported yet. Thus we prepared the spin labeled 7 acridono- and the spin
labeled 8 acridino-macrocycles.
studies of
these double sensors are of great importance. [15] Double sensors containing a crown ether part have not been
reported yet. Thus we prepared the spin labeled 7 acridono- and the spin
labeled 8 acridino-macrocycles.
The complexation ability of spin labeled 7 acridono- and 8 acridino-crown ethers and their reduced analogues 9 and 10 towards some biologically important metal ions [Na(I), K(I), Mg(II), Ca(II), Zn(II)] was studied by fluorescence spectroscopy. [10] In the case of paramagnetic derivatives (7 and 8) fluorescence quenching occurred. Acridino-crown ethers formed high stability complexes with Ca(II) and Zn(II) (logK≈6.7), and crown ether 8 caused a large fluorescence enhancement with complexation of Ca(II) (Fig. 4).
Figure 4. Double labeled ligand 8 induced
large fluorescence-enhancement with Ca(II)
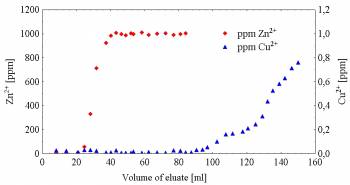 Selectivity of
the silica gel-bound acridino-18-crown-6 ether SP-11 toward some
biologically important metal ions was studied by stirring method. Stationary
phase SP-11 bound Ag(I), Cu(II) and Hg(II) ions strongly (logK≈2.0). Preliminary
experiments were also carried out for metal ion removal from dilute aqueous
solutions using column chromatography. Removal of Cu(II) ions in the amount of
10 ppm in the presence of 100 fold excess of Zn(II) ions in aqueous solution
could be achieved (Fig. 5). [9]
Selectivity of
the silica gel-bound acridino-18-crown-6 ether SP-11 toward some
biologically important metal ions was studied by stirring method. Stationary
phase SP-11 bound Ag(I), Cu(II) and Hg(II) ions strongly (logK≈2.0). Preliminary
experiments were also carried out for metal ion removal from dilute aqueous
solutions using column chromatography. Removal of Cu(II) ions in the amount of
10 ppm in the presence of 100 fold excess of Zn(II) ions in aqueous solution
could be achieved (Fig. 5). [9]
Figure 5. Chromatographic measures
with SP-11
Expected impact and further research
We synthesized new 18-crown-6 ether type achiral and enantiomerically pure sensor and selector molecules containing an acridino heterocyclic subunit. The complexation properties of these ligands toward different metal ions and enantiomers of protonated primary organic amines were studied using potentiometric, fluorescence spectroscopic, stirring and chromatographic methods. We published our results in high ranking international journals of our research field such as Tetrahedron and Tetrahedron:Asymmetry.
Regarding our further research, we would like to
attach other chains containing different functionalities to acridino-crown ethers
and also synthetize new macrocycles containing other heterocyclic subunits
(phenothiazine, for example) and study their selectivities toward biologically
important metal ions and the enantiomers of selected chiral molecules.
Publications, references, links
Publications
-
Kertész, J.; Huszthy, P.; Kormos, A.; Bertha, F.; Horváth, V.; Horvai, G. Tetrahedron: Asymm. 2009, 20, 2795–2801.
-
Kertész, J.; Móczár, I.; Kormos, A.; Baranyai, P.; Kubinyi, M.; Tóth, K.; Huszthy, P. Tetrahedron: Asymm. 2011, 22, 684–689.
-
Kertész, J.; Huszthy, P.; Kormos, A.; Bezúr, L. Tetrahedron 2011, 67, 5206–5212.
-
Kertész, J.; Bognár, B.; Kormos, A.; Móczár, I.; Baranyai, P.; Kubinyi, M; Kálai, T.; Hideg, K.; Huszthy, P. Tetrahedron. 2011, submitted to journal
References
[1] Atwood, J. L.;Steed, J. W. Encyclopedia of Supramolecular Chemistry. 2004, Marcel Dekker: New York, NY
[2] Steed, J. W.; Atwood, J. L. Supramolecular Chemistry. 2009, Wiley, 2nd ed.
[3] Pedersen, C. J., J. Am. Chem. Soc. 1967, 89, 2495–2496.
[4] Izatt, R. M.; Pawlak, K.; Bradshaw, J. S.; Bruening, R. L. Chem. Rev. 1991, 91, 1721–2085.
[5] Izatt, R. M.; Pawlak, K.; Bradshaw, J. S. Chem. Rev. 1995, 95, 2529–2586.
[6] Zhang, X. X.; Bradshaw, J. S.; Izatt, R. M. Chem. Rev. 1997, 97, 3313–3361.
[7] Kertész, J.; Huszthy, P.; Kormos, A.; Bertha, F.; Horváth, V.; Horvai, G. Tetrahedron: Asymm. 2009, 20, 2795–2801.
[8] Kertész, J.; Móczár, I.; Kormos, A.; Baranyai, P.; Kubinyi, M.; Tóth, K.; Huszthy, P. Tetrahedron: Asymm. 2011, 22, 684–689.
[9] Kertész, J.; Huszthy, P.; Kormos, A.; Bezúr, L. Tetrahedron 2011, 67, 5206–5212.
[10] Kertész, J.; Bognár, B.; Kormos, A.;
Móczár, I.; Baranyai, P.; Kubinyi, M; Kálai, T.; Hideg, K.; Huszthy, P.
Tetrahedron 2011, submitted to journal
[11] Morf, W. E. The Principles of
Ion-Selective Electrodes and of Membrane Transport. Elsevier: New York,
1981, p. 264–336.
[12] Koryta, J.; Štulík, K. Ion-selective
Electrodes. 2nd ed., Cambridge University Press, Cambridge, 1983, p.
168–194.
[13] Valeur, B. Molecular Fluorescence:
Principles and Applications. Wiley-VCH: Weinheim, Germany, 2002.
[14] Lakowicz, J. R. Principles of Fluorescence Spectroscopy. 3rd ed.; Springer Science+Business Media: New York, NY, 2006.
[15] Kálai, T. Doctor of HAS theses
2007.

