|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisor: Dr. KUPAI József
Development of continuous technologies based on twin-screw granulation
Introducing the research area
The pharmaceutical industry faces a major paradigm change with the spread of continuous manufacturing (CM) technologies. Although continuous processes are widespread in other sectors of the chemical industry with numerous well-documented advantages (high productivity, robust and agile manufacturing, increased energy- and cost efficiency, and simple scale-up), the pharmaceutical field is still mainly dominated by traditional batch processes. The shift is supported by the regulatory agencies and big pharmaceutical industries, which resulted in several commercially available drug products formulated using continuous technologies. More research is needed, however, to fully realize the potential of CM and the further spreading of these technologies. [1, 2] A promising continuous technology is twin-screw granulation, the continuous alternative of granulation, a basic and widely used formulation technique. The goal of my research is the development of the twin-screw granulation process to advance the industrial application of the technology. My work focuses on a deeper understanding of the granulation process, its integration with other continuous technologies, and the assurance of product quality.
Brief introduction of the research place
One of the main goals of the FirePharma research group led by Dr. Nagy Zsombor is to aid the modernization of the pharmaceutical sector. This is carried out by the development of innovative, new pharmaceutical processes and effective, reliable quality control systems. The relevance of the research topics is illustrated by the numerous industrial collaborations with several important Hungarian and international pharmaceutical companies.
History and context of the research
Granulation is a well-known and widely used particle agglomeration process that enhances the physical properties of powders, including improved flowability, uniformity, and tabletability. As tablets are the most popular dosage forms, and most tableting processes contain a granulation step, the importance of the method in the pharmaceutical field is inevitable. Although the continuous alternative of the technology, the twin-screw granulation, has numerous advantages and could easily substitute the currently used batch process, its widespread application is yet to come. [3]
Ensuring the proper product quality is a great challenge in the pharmaceutical sector. Therefore, the twin-screw process also needs to be supplemented with effective quality assurance systems. As with all continuous processes, the twin-screw granulation can be more efficiently monitored with modern in-line process analytical technologies(PAT) compared to traditional offline methods. These systems follow the critical product qualities in real time; therefore, potential errors can be detected, and a higher level of quality can be assured. [4] Moreover, the large amount of collected data can be further analyzed, leading to a deeper process understanding, and can also be utilized for the modeling of the process. [5]
The great potential of CM can be realized via the twin-screw granulation process, as the demand for the granulation technology is high, and the method possesses all the advantages of continuous processes. To reach extensive industrial applications, linking technology with effective quality assurance systems is crucial.
The research goals, open questions
Although more research focuses on continuous pharmaceutical processes, significantly less details integrated systems, where more technologies are operated connectedly. Therefore, the goal of my research was to develop integrated, connected continuous systems based on twin-screw granulation to aid the industrial application of the process. Within this framework, the flowability and compressibility of several – both placebo and active pharmaceutical ingredient (API)-containing – powder mixtures were enhanced with twin-screw granulation. I aimed to develop and investigate a fully continuous powder-to-tablet line. Different types of granulations (wet and melt granulation) were evaluated, with an emphasis on the understanding of the course of the granulation process and the examination of the effects of the process parameters.
As the assurance of adequate product quality is crucial in the pharmaceutical field, an important aspect of my work was the development of PAT tools and their combination with the continuous system. My first goal was the in-line, real monitoring of a critical product quality, the moisture content of the granules with a near-infrared (NIR) spectra-based method. My next aim was the development of an even more advanced monitoring system, where the moisture content could be predicted indirectly. The goal was to construct artificial neural network(ANN)-based models that can determine the moisture content merely from the collected process parameters.
Methods
A fully continuous, connected powder-to-tablet line was developed. The system comprised of a gravimetric feeder, a twin-screw wet granulator, a continuous horizontal fluid bed dryer, a continuous oscillating milling device, a vibratory conveying feeder, and a continuous tableting machine.
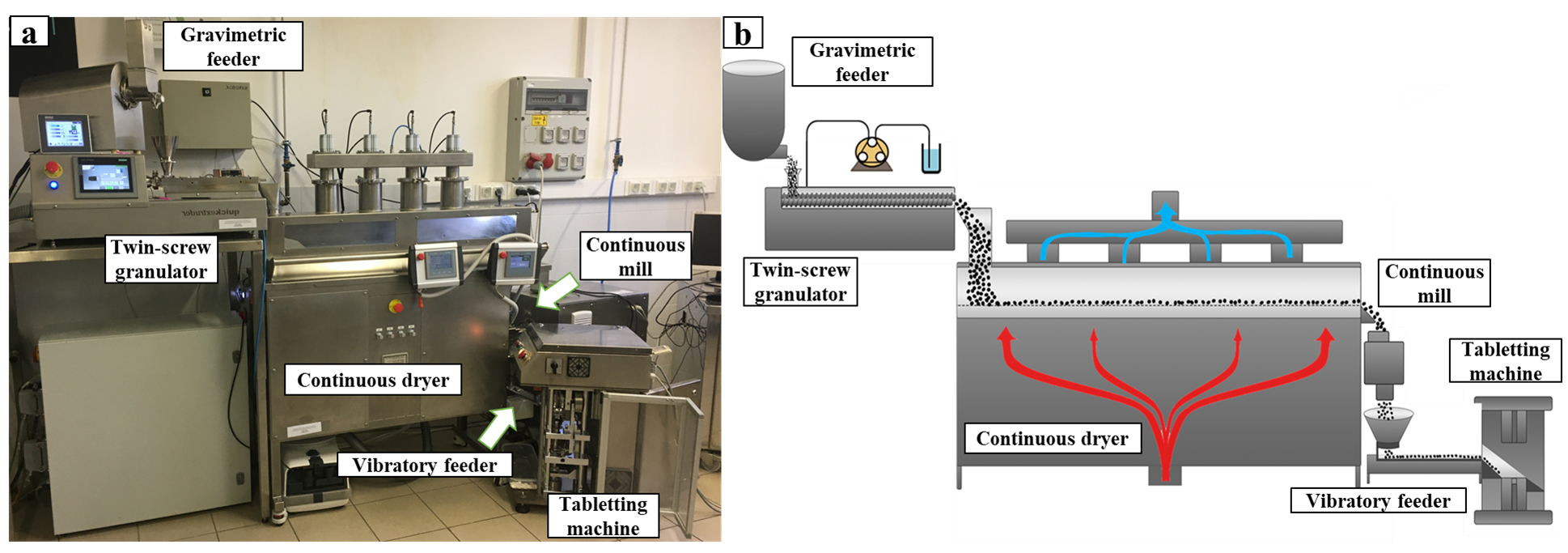
Fig. 1: The (a) picture and the (b) semantic drawing of the continuous system.
Melt granulation
The line was first applied for the melt granulation of caffeine while using polyethylene-glycol (PEG) as a binder. During the process, the PEG melted inside the twin-screw equipment, aiding the granule formation, thus the addition of any liquid could be avoided. In this case, the dryer was used with cold air to cool down the hot granules to room temperature. The effect of the process parameters was evaluated, and the scale-up was also achieved. The scale-up was carried out in the same system (without the use of new equipment) by only increasing the throughput.
Wet granulation and in-line process monitoring
Next, the wet granulation of glucose-monohydrate was investigated with the same line. In this case, a peristaltic pump was also added to the system for the feeding of the granulation liquid (distilled water). The effect of the process parameters – including the screw configuration – was also evaluated. The moisture content of the granules was monitored in-line in real-time with NIR spectroscopy and a spectra-based model using multivariate data analysis – more specifically with the partial least squares (PLS) method. Samples were collected during the process to validate the model with offline loss-on-drying measurements.
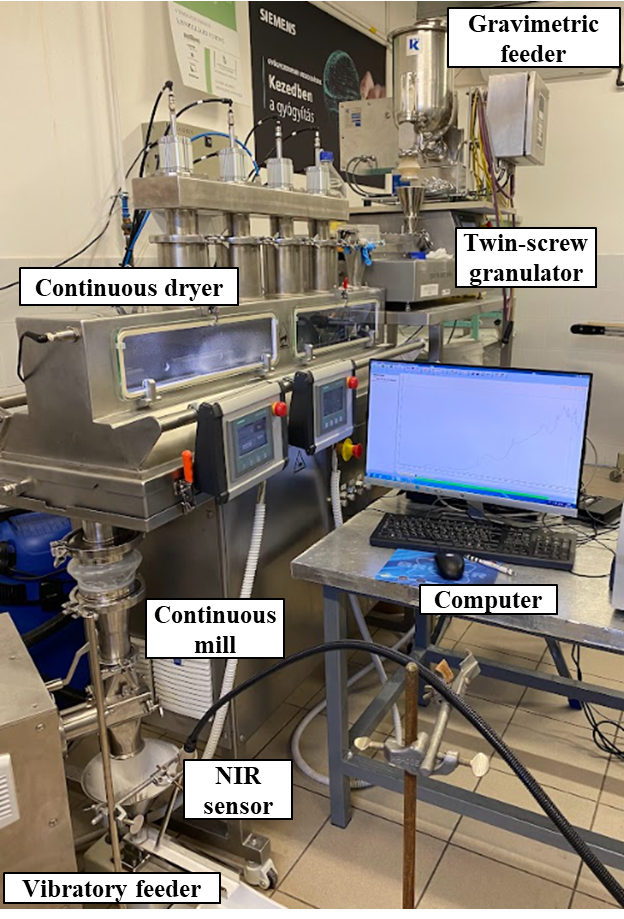
Fig. 2: The in-line monitoring of the process.
Development of a predictive model
The moisture content was also monitored with a similar NIR spectra-based PLS model during the wet granulation of a placebo system. In this case, however, the process parameters (mass flow, drying temperature, liquid-to-solid ratio) were also collected with SIMATIC SIPAT software. To investigate the relationship between these parameters and the moisture content, an ANN model was developed in the Matlab program. On this basis, a model was created that can estimate the moisture content from the fixed process parameters without direct measurement. A model was built with 107 data points (moisture contents with the related process parameters), with 80% used to train the model and 20% to validate it.
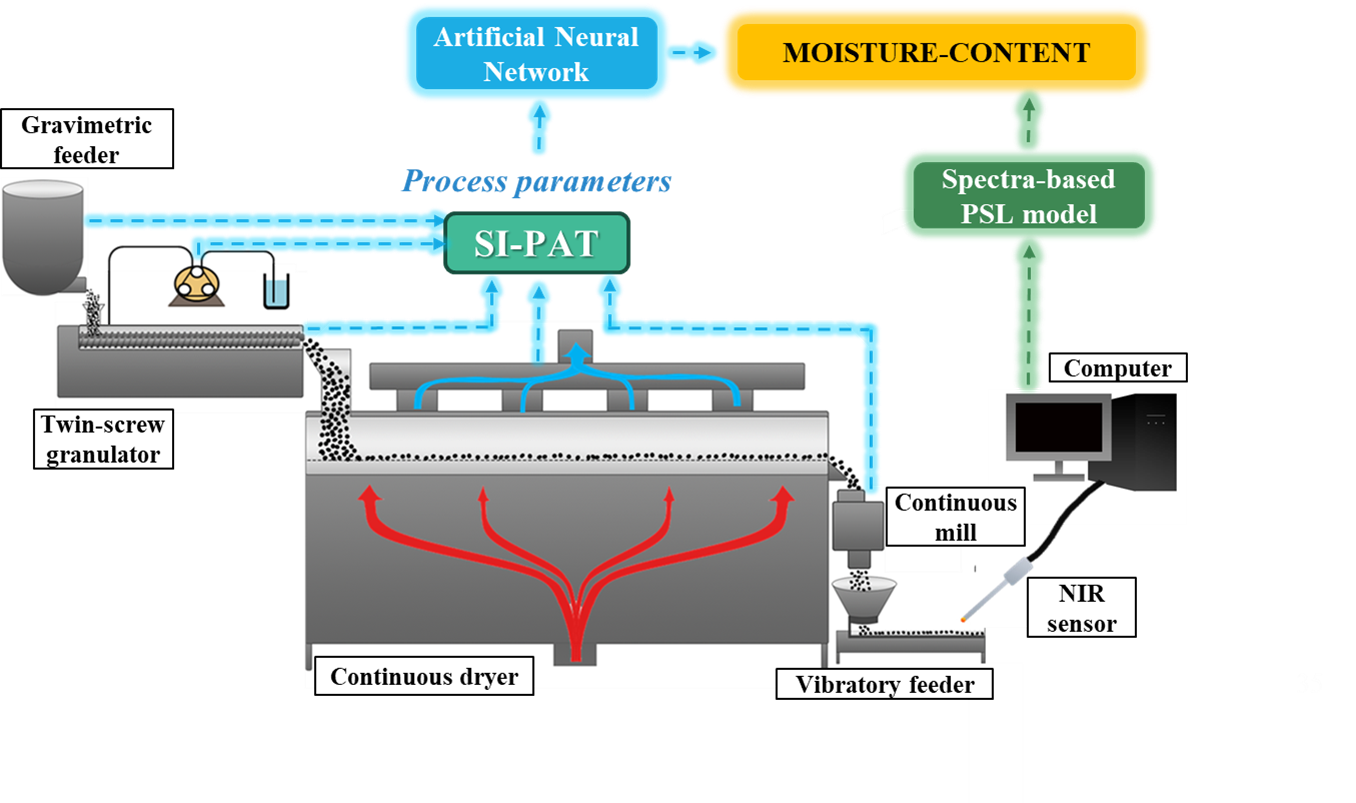
Fig. 3: The monitoring of the moisture content with a NIR spectra-based PLS model (green) and an ANN-based predictive model (blue).
Investigation of the granule and tablet properties
The morphology of the granules and the initial powders were investigated with scanning electron microscopy, the particle size distribution was measured with laser diffraction, furthermore, the flowability was also determined. The mechanical properties of the tablets (breaking force, friability) were also evaluated together with the dissolution properties.
Results
A fully connected continuous, powder-to-tablet line was developed and utilized for both wet and melt granulation. The flowability and tabletability of several powders were significantly enhanced, while the effects of the process parameters were evaluated to ensure proper operation.
During melt granulation, the average particle size of the caffeine-containing powder blend increased, and its flowability properties changed from the “very bad” category to “good”. The tabletability also improved: the tablets pressed from the initial powder were very fragile, but the granulation increased the breaking force (from below 40 N to over 80 N) and decreased the friability loss (under 0.3%), making the mechanical properties adequate.
Fig. 4: The difference between “very bad” and “good” flowability.
The scale-up was also successful, up to 16 times the initial production speed (from 0.5 kg/h to 8 kg/h). It was conducted without any difficulty, which is a great advantage, being the scale-up usually a particularly challenging step in case of batch production, as the use of new equipment (with often different geometry) can require a completely new optimization. This could be avoided with our system while reaching productivity that could be even suitable for industrial operation.
The system was also successfully used for wet granulation, and the flowability and compressibility of the glucose were enhanced. It was revealed that the screw configuration with increased pressure inside the equipment was more suitable for this system, as it leads to the breakage of the glucose crystalline, preventing the formation of weak spots inside the tablets. With our process, tablets with increased breaking force and, thus, adequate mechanical properties could be produced.

Fig. 5: the improvement of the properties during the granulation process.
The in-line, real-time process monitoring with the NIR spectra-based PLS model was also achieved. The model could determine the moisture content accurately, confirmed by the similar results of the in-line and offline measurements. With this model, the moisture content could be monitored during the process, therefore the drying temperature leading to the target (8%) moisture-content, could be easily revealed. Moreover, later, the quality of the granules could also be assured with continuous monitoring.

Fig. 6: The in-line monitoring of the moisture content.
The moisture content was also successfully predicted with the ANN-based model. The values determined only from the collected operation parameters were remarkably close to the results of the in-line and offline measurements, indicating that the model was dependable. In the future (after the implementation of some further improvements), this predictive model could even replace these measurements, leading to a highly advanced quality assurance system and reducing the cost significantly.
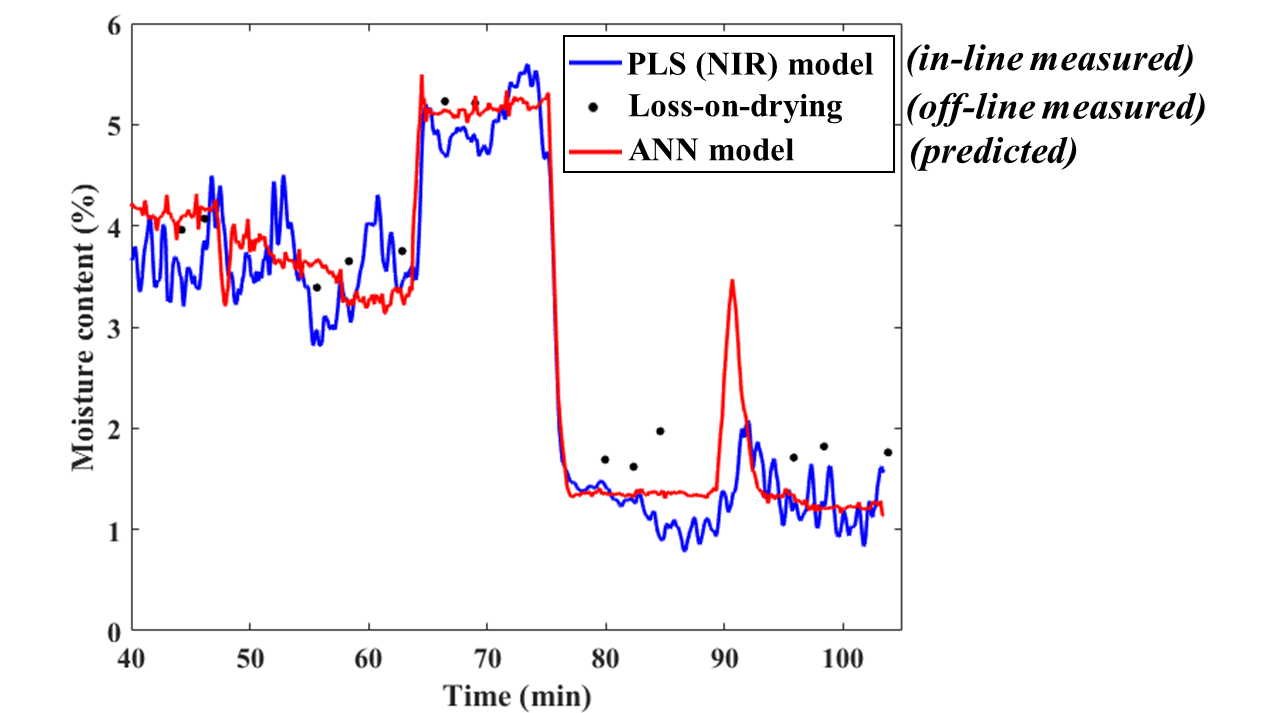
Fig. 7: The predicted moisture content from the ANN-based model compared to the results of the in-line and offline measurements.
Expected impact and further research
I developed a fully connected, continuous system that can be used for wet and melt granulation to successfully enhance the physical properties of several powder mixtures. The in-line monitoring of the system was also accomplished with both a spectra-based, and a predictive model to ensure the proper product quality. The results of my research illustrate the many advantages of CM and can aid the industrial application of the twin-screw granulation process. The scientific work was reached with the sponsorship of the Gedeon Richter Talentum Foundation in the framework of the Gedeon Richter Excellence PhD Scholarship of Gedeon Richter, which further shows the increased industrial interest in the topic.
Publications, references, links
List of corresponding own publications
[1] Petra Záhonyi; Edina Szabó; András Domokos; Anna Haraszti, Martin Gyürkés; Erzsébet Moharos; Zsombor K. Nagy, Continuous integrated production of glucose granules with enhanced flowability and compressibility, International Journal of Pharmaceutics (2022)
[2] Petra Záhonyi, Fekete Dániel, Edina Szabó, Lajos Madarász, Árnika Fazekas, Anna Haraszti, Zsombor K Nagy, Integrated continuous melt granulation-based powder-to-tablet line: process investigation and scale-up on the same equipment, European Journal of Pharmaceutics and Biopharmaceutics (2023)
[3] Martin Gyürkés, Lajos Madarász, Petra Záhonyi, Ákos Köte, Brigitta Nagy, Hajnalka Pataki, Zsombor Kristóf Nagy, András Domokos, Attila Farkas, Soft sensor for content prediction in an integrated continuous pharmaceutical formulation line based on the Residence Time Distribution of unit operations, International Journal of Pharmaceutics, (2022)
[4] Edina Szabó, Anna Haraszti, Petra Záhonyi, Dániel Vadas, István Csontos, Zsombor Kristóf Nagy, Guy Van den Mooter, György Marosi, Evaluation of Different Thermoanalytical Methods for the Analysis of the Stability of Naproxen-Loaded Amorphous Solid Dispersions, Pharmaceutics (2022)
[5] Panna Vass, András Domokos, Eszter Pantea, Botond Szilágyi, Mónika Molnár, Petra Záhonyi, Brigitta Nagy, Zsombor Kristóf Nagy, Processing of thermosensitive biological API from suspension using an integrated continuous granulation – Drying – Milling line into powder ready for tableting, Drying Technology (2022)
[6] Martin Gyürkés, Lajos Madarász, Petra Záhonyi, Ákos Köte, Brigitta Nagy, Hajnalka Pataki, Zsombor Kristóf Nagy, András Domokos, Attila Farkas, Soft sensor for content prediction in an integrated continuous pharmaceutical formulation line based on the residence time distribution of unit operations International Journal of Pharmaceutics (2022)
[7] Edina Szabó, Petra Záhonyi, Dorián L Galata, Lajos Madarász, Panna Vass, Attila Farkas, Jens Dhondt, Sune K Andersen, Tamás Vígh, Geert Verreck, István Csontos, György Marosi, Zsombor K Nagy, Powder filling of electrospun material in vials: A proof-of-concept study, International Journal of Pharmaceutics (2022)
[8] Szabó Edina, Záhonyi Petra, Brecska Dániel, Galata Dorián L., Mészáros Lilla A., Madarász Lajos, Csorba Kristóf, Vass Panna, Hirsch Edit, Szafraniec-Szczęsny Joanna, Csontos István, Farkas Attila, Van denMooter Guy, Nagy Zsombor K., Marosi György, Comparison of Amorphous Solid Dispersions of Spironolactone Prepared by Spray Drying and Electrospinning: The Influence of the Preparation Method on the Dissolution Properties, Molecular Pharmaceutics (2021)
[9] Szabó Edina, Záhonyi Petra, Gyürkés Martin, Nagy Brigitta, Galata Dorián L., Madarász Lajos, Hirsch Edit, Farkas Attila, Andersen Sune K., Vígh Tamás, Verreck Geert, Csontos István, Marosi György, Nagy Zsombor K., Continuous downstream processing of milled electrospun fibers to tablets monitored by near-infrared and Raman spectroscopy, European Journal of Pharmaceutical Sciences (2021)
Table of links
process analytical technologies
List of references
[1] S.L. Lee, T.F. O’Connor, X. Yang, C.N. Cruz, S. Chatterjee, R.D. Madurawe, C.M.V. Moore, L.X. Yu, J. Woodcock, Modernizing Pharmaceutical Manufacturing: from Batch to Continuous Production, J. Pharm. Innov. 10 (2015) 191–199.
[2] András Domokos, Brigitta Nagy, Botond Szilágyi, György Marosi, and Zsombor Kristóf Nagy, Integrated Continuous Pharmaceutical Technologies—A Review, Org. Process Res. Dev. 2021, 25, 4, 721–739.
[3] C. Portier, C. Vervaet, V. Vanhoorne, Continuous twin screw granulation: A review of recent progress and opportunities in formulation and equipment design, Pharmaceutics. 13 (2021).
[4] Margot Fonteyne, Jurgen Vercruysse, Fien De Leersnyder, Bernd Van Snick, Chris Vervaet, Jean Paul Remon, Thomas De Beer Process Analytical Technology for continuous manufacturing of solid dosage forms, TrAC Trends in Analytical Chemistry, Volume 67, April 2015, Pages 159–166
[5] N.S. Arden, A.C. Fisher, K. Tyner, L.X. Yu, S.L. Lee, M. Kopcha, Industry 4.0 for pharmaceutical manufacturing: Preparing for the smart factories of the future, Int. J. Pharm. 602 (2021)
