|
|
BMe Research Grant |
|
Doctoral School of Electrical Engineering
BME-VIK, Department of Electronics Technology
Supervisor: Dr. MEDGYES Bálint
Electrochemical corrosion assessment of novel low-Ag micro-alloyed lead-free solder alloys
Introducing the research area
Today's electronic device materials are becoming less toxic and more miniaturized. For almost every electronic device, the question arises: can the functionality be implemented in smaller dimensions? It is worth noting that advancements in packaging technology have spurred a trend toward miniaturization, enabling the production of progressively smaller electronic devices with higher power density [1]. As a result, the heat generated by these devices during operation can potentially alter or degrade the natural oxide film, weakening its ability to protect the Sn-based lead-free substrate. Elevated-temperature aging speeds up the dehydration process, initiated at the oxide/solder interface and extending outward through the oxide film layer. This inevitably alters the stoichiometry and conductivity of the oxide film, affecting its corrosion resistance in different ways. Additionally, the high-temperature aging triggers oxidation in the pristine Sn-based lead-free substrate, complicating the changes in composition, thickness, and structure of the oxide film [1].
Brief introduction of the research place
My research was conducted at the Department of Electronics Technology (ETT) within BME, under the supervision of Dr. Bálint Medgyes. Within our department, we engage in active research focused on soldering technology. This includes assessing the resistance of various materials to electrochemical corrosion, analyzing the composition of corrosion products to understand failure mechanisms in electronics, and utilizing simulation and applied materials science techniques to investigate different failure mechanisms. For this reason, our research center is equipped with special equipment that can investigate solid-phase conductive, insulating, and semiconductor structures and, in some cases, can even carry out liquid-phase measurements between all scales from human visibility to nano sizes. For these purposes, the department has optical microscopes (Olympus SZX9 and BX51), an X-ray microscope (Dage XiDAT 6600), a scanning electron microscope (FEI Inspect S50) with an energy dispersive X-ray spectrometer unit (Bruker Quanta EDX), a scanning acoustic microscope (Sonix HS 1000), X-ray micro fluorescence analyzer (Spectro Midex M), and an atomic force microscope (Veeco diInnova). To perform investigations under harsh environments, different environmental chambers can be applied; a Highly Accelerated Stress Test chamber (Tabai Espec); two Thermal-Humidity Chambers (EHS-211M and Weiss, WK 180/40), and a Thermal Shock Chamber (Tabai Espec, TSE-11-A). For the electrochemical corrosion (ECC) measurements, we have an Electrochemical workstation (Voltalab PGZ 301 potentiostat) to conduct linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS).
History and context of the research
Corrosion represents the inherent deterioration of metallic materials, including both metals and alloys, leading to significant economic and safety repercussions [2]. In the field of electronics technology, ECC is an unavoidable issue stemming from the diverse phases present in the solder alloys utilized within electronic systems. Lead-containing solders (Sn-Pb) have been extensively employed in electronics manufacturing for many years due to their desirable soldering characteristics, including strong mechanical properties, excellent surface wetting behavior, low melting point, good resistance to corrosion, and affordability [3]. Nevertheless, the use of lead, classified as a heavy metal, has been prohibited for soldering purposes. Hence, there is a pressing requirement to examine the reliability factors associated with various Sn-based lead-free solder alloys that have been proposed as alternatives to Sn-Pb solder alloys [3].
Among these candidates, solder alloys containing tin (Sn), silver (Ag), and copper (Cu) (SAC) have emerged as promising contenders because of their improved reliability characteristics. However, the ECC is still inevitably present with SAC alloys, as it arises from the galvanic coupling due to the difference in standard electrode potential (E₀) between the β-Sn and intermetallic compound (IMC) phases (i.e., Ag3Sn and Cu6Sn5) [4]. Therefore, several techniques have been employed to enhance the corrosion resistance of SAC alloys, these include but are not limited to appending SAC alloys with more alloying elements [5] or nanoparticles [6], controlling the cooling process during solidification [7], and obtaining a native oxide film on SAC solder surface with better corrosion resistance [8]. It is worth noting that the last method is regarded as state-of-the-art in corrosion mitigation methods as the native oxide film on the surface acts as a protective barrier before the galvanic corrosion caused by the interaction between the Ag3Sn phases and the β-Sn matrix occurs, delaying the corrosion degradation of the solder substrate.
The research goals, open questions
In my research, I seek to answer the question of the effect of reducing Ag content on the long-term ECC behavior of SAC alloy. Therefore, I compare the ECC behavior of SAC305 solder alloy with SAC0307-1Bi-xMn (x = 0.1, 0.4, 0.7 wt.%) low-Ag content micro-alloyed lead-free solder alloys over 3 days immersion in 3.5 wt.% NaCl solution. A critical parameter for the advantageous long-term ECC behavior is the ability of the given solder alloy to develop a native surface oxide film immediately after polishing. However, to characterize the native oxide film, its composition and structure should be investigated where the former contains information about the oxides that constitute the layers of the film while the latter contains information about the uniformity, thickness, the integrity of the oxides, and the existence of the defects within the oxide film.
Methods
For the sample preparation process, I brought the lead-free alloys SAC-1Bi-xMn (x = 0.1, 0.4, 0.7 wt.%) and SAC305 in flux-free solder wires form with a 1 mm diameter. To set up the working electrode, the solder wires underwent rinsing with distilled water and ethanol, followed by embedding in silicon tubes using epoxy resin for 2–3 days until fully hardened. This process left an exposed surface area of around 0.785 mm2 as depicted in Figure 1. Before conducting electrochemical tests, the working electrodes underwent polishing using silicon carbide (SiC) abrasive papers ranging in grades from 220 to 4000, resulting in a relatively smooth working electrode surface.
Figure 1. Working electrode preparation by embedding the solder wire in epoxy.
For the ECC behavior testing, two types of electrochemical measurements were conducted: (i) LSV and (ii) EIS. LSV tests for the short-term ECC behavior were implemented in a single-compartment cell utilizing a standard three-electrodes setup of Ag/AgCl/3 M KCl as the reference electrode, a platinum net used as a counter electrode, and the sample as a working electrode. Measurements were performed in 3.5 wt.% NaCl solution at room temperature. Polarization measurements were performed in different potential ranges, most often from − 100 mV vs. open circuit potential (OCP) to 1000 mV vs. Ag/AgCl reference electrode at a scan rate of 10 mV/min. For the long-term ECC behavior evaluation, I compiled a series of measurements in which the OCP and EIS measurements were repeated cyclically. Impedance spectra were recorded at OCP using a 10 mV effective amplitude sinusoidal signal with 10 points per decade over frequencies ranging from 10 kHz to 10 MHz. During the monitoring of the time dependence of corrosion, a waiting time of 30 min was applied between recording individual impedance spectra, during which the OCP was measured.
To achieve systematic investigations regarding the structure and composition, high-resolution transmission electron microscopy (HR-TEM) and X-ray photoelectron spectroscopy (XPS) are required due to the nano nature of the native oxide film on SAC alloys.
Results
Figure 2 shows the corrosion potential (Ecorr) and corrosion current density (icorr) values extracted from the LSV test; these results illustrate that there were no significant corrosion rate differences between SAC305 and SAC0307-Bi-xMn in the case of short-term measurements. This might be attributed to the fact that the surface of the solder alloys in the initial stage is covered with a thin native passive oxide layer that developed after fresh polishing, which is dominantly determined by the main component (i.e., Sn) in the alloys.
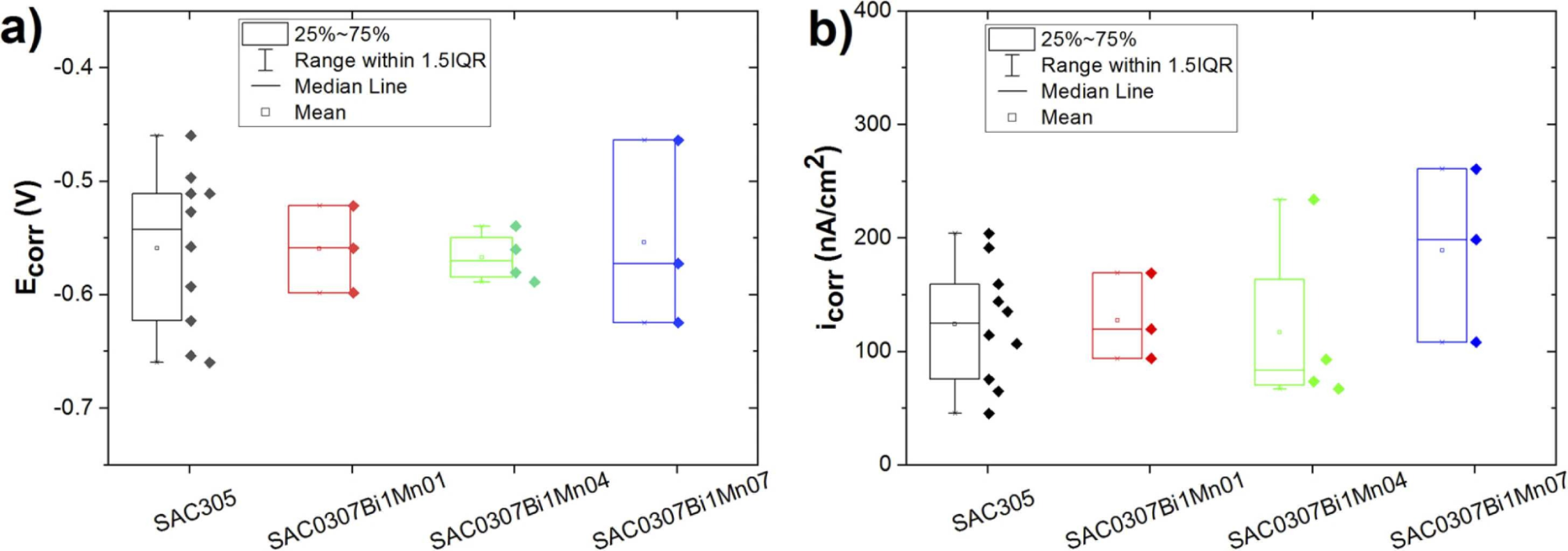
Figure 2. Box plot of (a) Ecorr and (b) icorr values determined by the Tafel extrapolation for the tested alloys in 3.5 wt.% NaCl solution.
For the long-term ECC behavior, Figure 3 illustrates the temporal changes in the polarization resistance of the solder alloys when exposed to a 3.5 wt.% NaCl solution following three sets of OCP and EIS measurements. It is evident that all alloys underwent localized corrosion, indicating their vulnerability to such corrosion in the NaCl solution. However, their resistance to localized corrosion varies. For instance, SAC305 displayed an earlier onset of localized corrosion, beginning after 4–18 hours (Fig. 3a), while SAC0307–1Bi-xMn solders exhibited a delayed onset of localized corrosion, commencing after 14–40 hours (Fig. 3b-d), indicating their more favorable long-term behavior against ECC in the NaCl solution. Specifically, SAC0307–1Bi-0.1Mn showed localized corrosion onset after 25–28 hours, SAC0307–1Bi-0.4Mn solder exhibited it after 14–28 hours, and for SAC0307–1Bi-0.7Mn solder, it occurred after 18–39 hours.
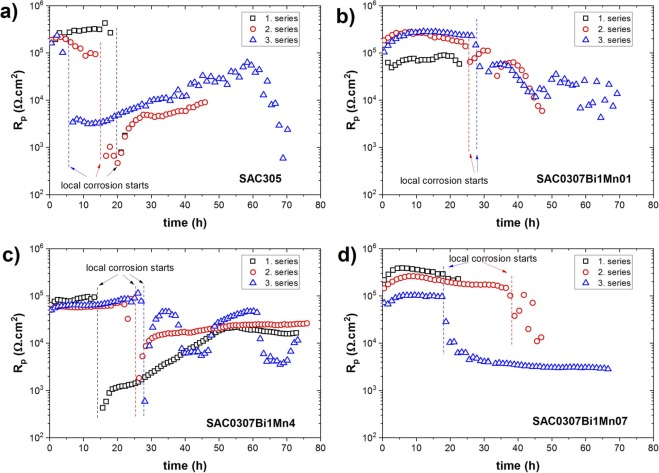
Figure 3. Local corrosion commencement monitoring and variation of polarization resistance values for the solder alloys in 3.5 wt.% NaCl solution.
However, to verify the effect of reducing Ag content on the long-term ECC behavior of SAC alloys, Figure 4 illustrates the temporal changes in the polarization resistance of SAC305 and SAC0307 solder alloys in 3.5 wt.% NaCl.
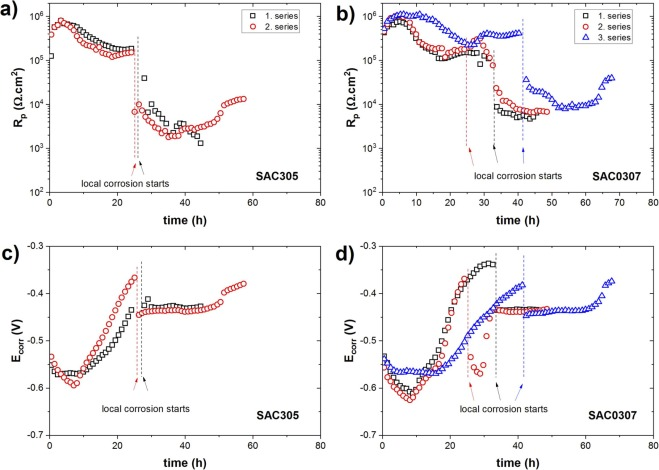
Figure 4. Local corrosion commencement monitoring and variation of polarization resistance and corrosion potential values for SAC305 and SAC0307 solder alloy electrodes made of paste by reflowing.
it can be seen that in the case of the SAC0307 solder alloy with reduced silver content, local corrosion starts later, after 24, 31, and 40 h (mean value: 32 h) while for SAC305 it starts after 24–26 h (mean value: 25 h). The advantageous effect of reducing Ag content in SAC alloys on their ECC behavior could stem from one or both of the following factors: reducing the micro-galvanic coupling between the Ag3Sn IMC phase and β-Sn phase, and/or decreasing the metallic Ag content within the native passive oxide film, thus preserving the integrity of the film. Furthermore, the effect of alloying elements such as Bi and Mn might play a role in enhancing corrosion resistance by contributing to refining the microstructure of the solder alloy and/or integrity of the native oxide film.
Expected impact and further research
The research has shown the advantageous effect of implementing “Lead-free Micro-alloyed low Ag Solder Alloys” from the perspective of ECC. Therefore, it is believed that these findings (published in an international peer-reviewed journal (D1/Q1 level)) motivate us to investigate the effect of implementing this type on other reliability aspects. Furthermore, it is worth noting that the cost of micro-alloyed SAC is 5–10% lower compared to the commonly used SAC305 alloy. However, I would like to take the current research to the next level by developing the native oxide film on the solders by heat treatment (e.g., 90 °C for 2, 4, 6, and 8 h) and then conducting another ECC evaluation. This is an important step that simulates the ECC performance under excessive heat release by the electronic device itself during operation which results from the miniaturization trend in packaging technology.
Publications, references, links
Gharaibeh Ali (Ali Gharaibeh) (MTMT)
List of corresponding own publications.
International, peer-reviewed journal papers, written in English.
[L1] B. Medgyes, A. Gharaibeh, G. Harsányi, B. Pécz, and I. Felhősi, “Electrochemical corrosion and electrochemical migration characteristics of SAC-1Bi-xMn solder alloys in NaCl solution,” Corros. Sci., vol. 213, no. January, p. 110965, Apr. 2023, doi: 10.1016/j.corsci.2023.110965.
International, peer-reviewed conference papers, written in English.
[R1] A. Gharaibeh and B. Medgyes, "Electrochemical Corrosion Assessment of Low-Ag SAC Lead-Free Solder Alloys," 2023 IEEE 29th International Symposium for Design and Technology in Electronic Packaging (SIITME), Craiova, Romania, 2023, pp. 127-131, doi: 10.1109/SIITME59799.2023.10430530.
I was awarded the "Excellent Presentation Award for Young Scientist."
Table of links.
Website of the Department of Electronics Technology (bme.hu)
List of references.
[1] C. Qiao, X. Sun, Y. Wang, L. Hao, X. Liu, and X. An, “High-temperature aging time-induced composition and thickness evolution in the native oxides film on Sn solder substrate,” J. Mater. Sci. Mater. Electron., vol. 32, no. 19, pp. 24209–24228, 2021, doi: 10.1007/s10854-021-06887-2.
[2] C. Verma, E. E. Ebenso, and M. A. Quraishi, “Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: A review,” J. Mol. Liq., vol. 248, pp. 927–942, 2017, doi: 10.1016/j.molliq.2017.10.094.
[3] S. Li et al., “Corrosion behavior of Sn-based lead-free solder alloys: a review,” J. Mater. Sci. Mater. Electron., vol. 31, no. 12, pp. 9076–9090, May 2020, doi: 10.1007/s10854-020-03540-2.
[4] A. Gharaibeh, I. Felhősi, Z. Keresztes, G. Harsányi, B. Illés, and B. Medgyes, “Electrochemical Corrosion of SAC Alloys: A Review,” Metals (Basel)., vol. 10, no. 10, pp. 1–18, 2020, doi: 10.3390/met10101276.
[5] F. Li, V. Verdingovas, K. Dirscherl, G. Harsányi, B. Medgyes, and R. Ambat, “Influence of Ni, Bi, and Sb additives on the microstructure and the corrosion behavior of Sn–Ag–Cu solder alloys,” J. Mater. Sci. Mater. Electron., vol. 31, no. 18, pp. 15308–15321, 2020, doi: 10.1007/s10854-020-04095-y.
[6] W. Jie, X. Songbai, W. Jingwen, W. Jianxin, and Y. Deng, “Enhancement on the high-temperature joint reliability and corrosion resistance of Sn–0.3Ag–0.7Cu low-Ag solder contributed by Al2O3 Nanoparticles (0.12 wt%),” J. Mater. Sci. Mater. Electron., vol. 29, no. 23, pp. 19663–19677, 2018, doi: 10.1007/s10854-018-0092-z.
[7] M. Wang, J. Wang, H. Feng, and W. Ke, “Effects of microstructure and temperature on corrosion behavior of Sn-3.0Ag-0.5Cu lead-free solder,” J. Mater. Sci. Mater. Electron., vol. 23, no. 1, pp. 148–155, 2012, doi: 10.1007/s10854-011-0552-1.
[8] C. Qiao et al., “Native oxide film powered corrosion protection of underlying Pb-free Sn solder substrate,” Corros. Sci., vol. 221, no. April, p. 111359, 2023, doi: 10.1016/j.corsci.2023.111359.
