|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisors: Dr. Pataki Hajnalka, Dr. Marosi György
Development of continuous crystallization technologies for processing a flow reaction mixture
Introducing the research area
It has never been so urgent to accelerate drug product development as it is in the current global situation. This demand could be served by continuous technologies that offer shorter and more flexible development and a more straightforward and agile scale-up process than the currently widely used batch technologies. Recognizing these advantages, prominent pharmaceutical manufacturers and regulatory authorities encourage the conversion of continuous operations. Consequently, several synthetic and formulation procedures have been accomplished in a continuous mode; however, only a few studies have dealt with the direct connection or the comparison of such technologies [1]. Accordingly, my work aimed to develop continuous crystallization technologies suitable for the direct processing of a flow reaction mixture and can even be combined with further formulation steps. In addition, I have characterized the quality of the products in detail, and through this, I compared the developed methods; thus, helping drug researchers to choose between technologies.
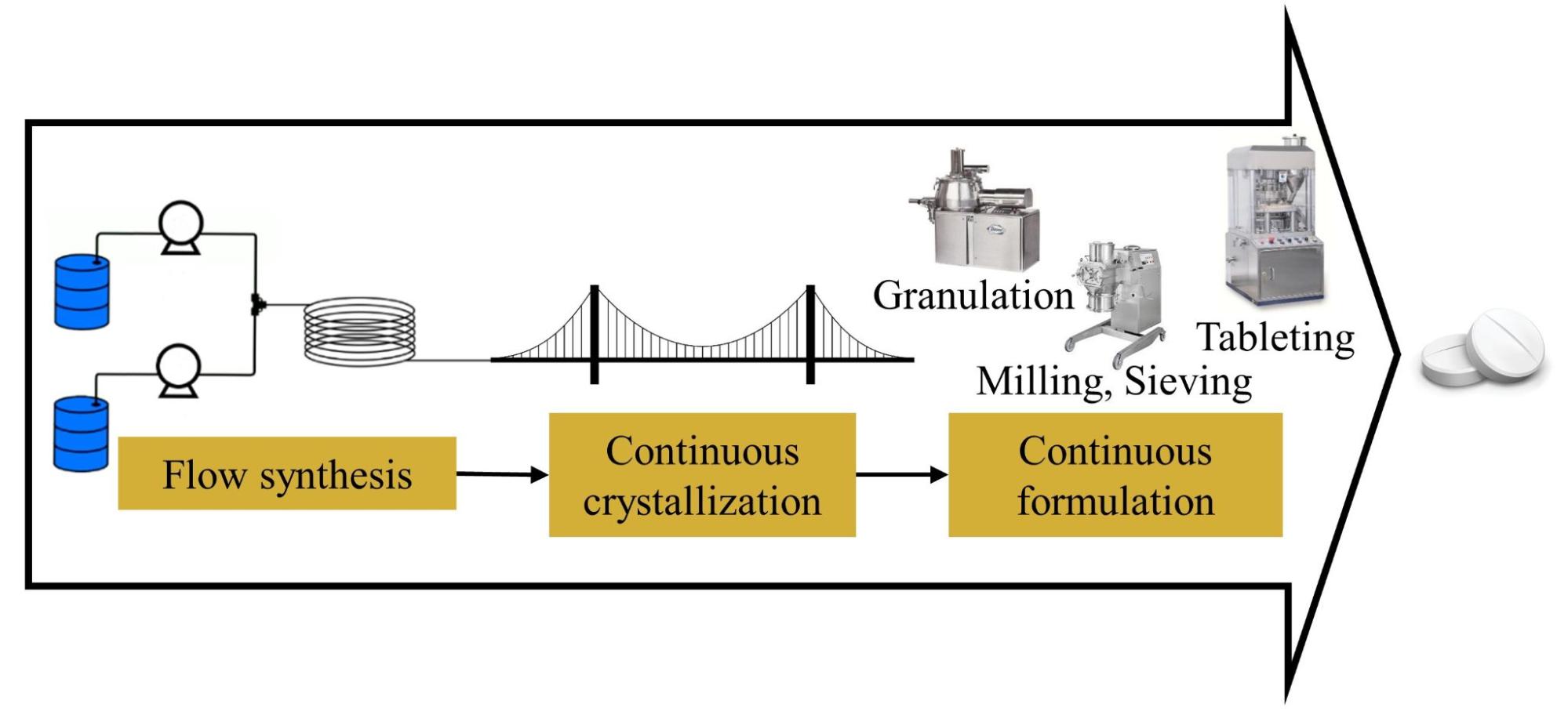
Figure 1 Continuous crystallization: the link between continuous synthesis and formulation.
Brief introduction of the research place
Our goals at FirePharma Research Group are to support the innovation of the pharmaceutical industry by developing novel continuous pharmaceutical technologies and connected real-time quality monitoring and control systems using state-of-the-art analytical and technological solutions. The industry collaborations of recent years prove that we now have excellent partnerships with numerous leading domestic and international pharmaceutical companies and collaborate with academic research teams.
History and context of the research
Crystallization, as the most widespread separation and purification method used in drug production, is a critical technology since the quantity and morphological properties of the product (polymorphism, crystal habit, crystal size, and crystal size distribution (CSD)) can be influenced via crystallization process parameters. Due to the morphology-determining characteristics of crystallization, the technological (flowability, compressibility) and biological (bioavailability) properties of the product can be fundamentally governed. Its significance in the pharmaceutical industry is also illustrated by the fact that more than 90% of small molecular drugs are marketed in crystalline form, and more than 80% of drug product manufacturing involves at least one crystallization step [2,3]. An innovative field of research is continuous technologies that can make production safer and more economical. Accordingly, in our research group, we work on developing continuous end-to-end (from synthesis to tableting) manufacturing alternatives, an essential part of which is developing, investigating, and understanding various continuous crystallization technologies [4,5].
Most of the published continuous crystallizations started from a clear solution of high drug concentration [6,7]. Processing the steady-state composition of a flow reaction mixture containing impurity byproducts is challenging as the number of variable crystallization parameters is reduced; therefore, only a few studies are concerned with this issue.
The research goals, open questions
My research aimed to develop various continuous crystallization methods for direct isolation and purification of acetylsalicylic acid (ASA, the active ingredient of Aspirin) from its multicomponent reaction mixture. This is to find methods that can be adjusted to the end-to-end production line of the ASA model drug, providing pure, homogeneous crystalline products for further formulation. For this purpose, I have applied and investigated four different self-built continuous crystallizer systems: (I.) an overflow mixed suspension mixed product removal (MSMPR) crystallizer, (II.) a triple impinging jet (TIJ) mixing element connecting to an overflow MSMPR crystallizer (TIJ-MSMPR), (III.) an ultrasonicated plug flow crystallizer (PFC), and (IV.) a connected ultrasonicated PFC and overflow MSMPR crystallizer system (PF-MSMPR) to perform an additive-assisted continuous crystallization. For comparability, the listed crystallization technologies were tested in a series of experiments applying similar setups.
Methods
The processed flow reaction mixture contained ASA active ingredient, a 5% salicylic acid (SA) impurity component, and multiple solvents such as ethyl acetate, acetic acid, or ethanol.
Four continuous crystallizer systems were applied to perform cooling-antisolvent crystallization of the ASA solution using multiple amounts (2-4-6x) of antisolvent for appropriate yield. (I.) The MSMPR crystallizer was equipped with an overflow tubing for pump-free and continuous withdrawal of the slurry without the mechanical degradation of the crystalline product. An inner vertical plate (baffle element) was placed into the crystallizer near the overflow tubing to avoid the immediate washing out of the fed solution and enable representative product removal. (II.) A TIJ mixer was designed to achieve efficient mixing even when the ratio of the solution and antisolvent streams was unequal. Therefore, in TIJ, two equal antisolvent streams collided with one ASA solution stream. The mixer was combined with an overflow MSMPR crystallizer to provide an appropriate crystallization time and hereby promote higher productivity.
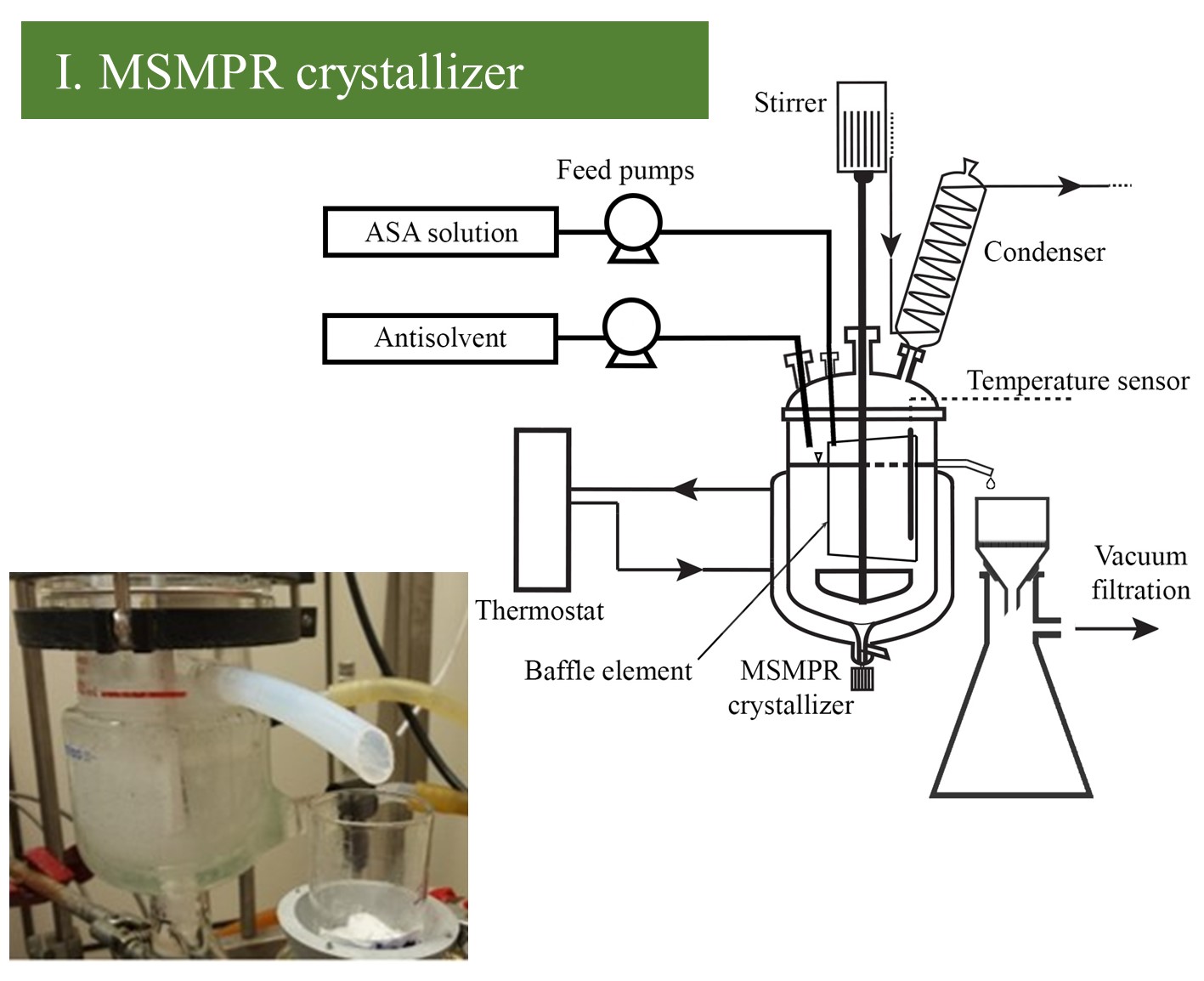
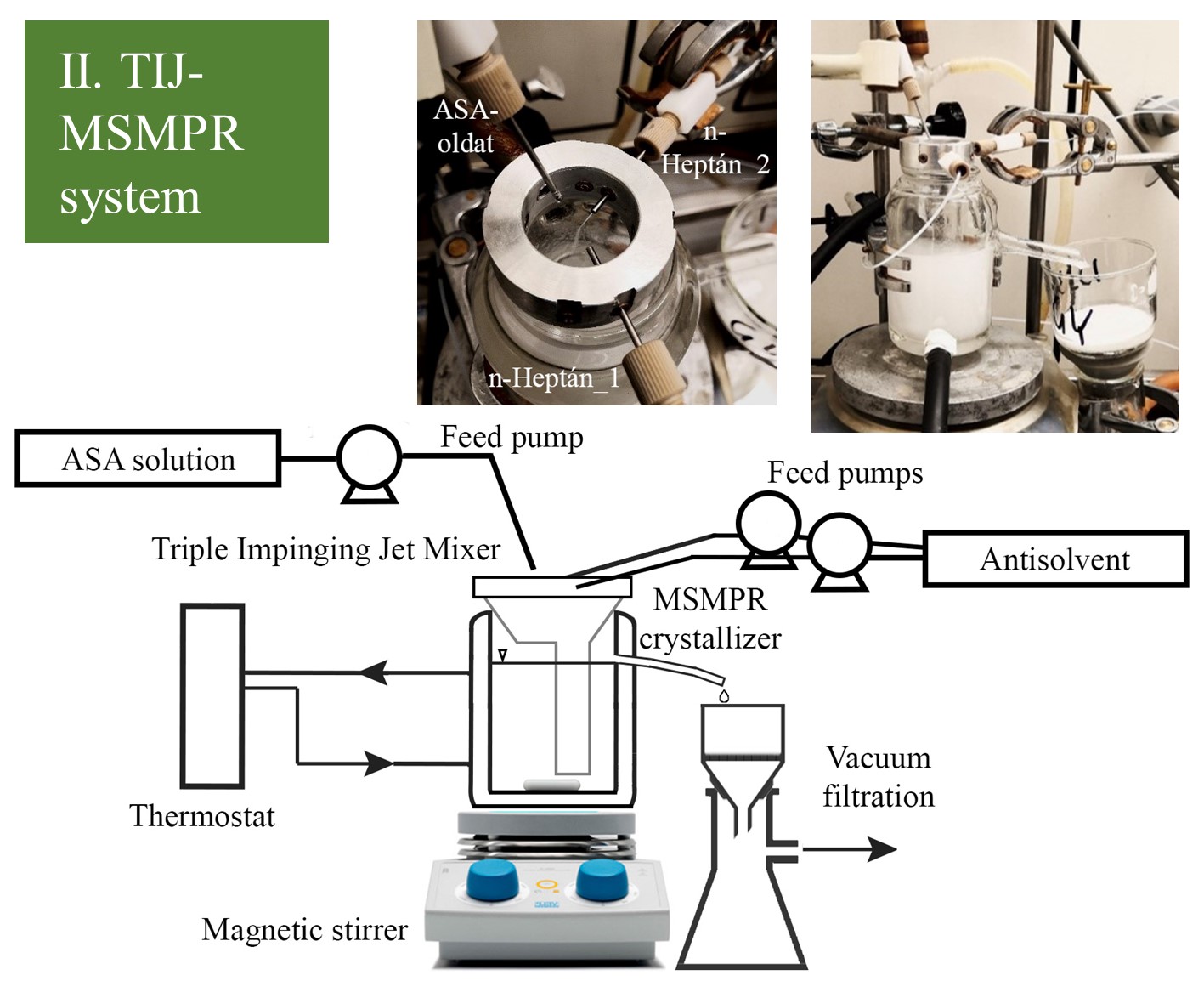
Figure 2 Schematic image and picture of MSMPR crystallizer and TIJ-MSMPR system.
(III.) The PFC crystallizer was equipped with a coaxial mixer that can be efficiently used in highly supersaturated, unstable systems where traditional mixing elements are rapidly blocked. Ultrasonication was applied to initiate homogeneous nucleation and prevent the clogging of crystallizer tubing. (IV.) The connected PF-MSMPR system was utilized to perform additive-assisted crystallization to enhance product morphology, improving the technological properties (flowability) of a small particle-sized product. For this purpose, the slurry prepared in the above-described ultrasonicated PFC was fed to an overflow MSMPR crystallizer in parallel with a 1, 7.5, or 14% polyvinylpyrrolidone K30 (PVP-K30) additive.
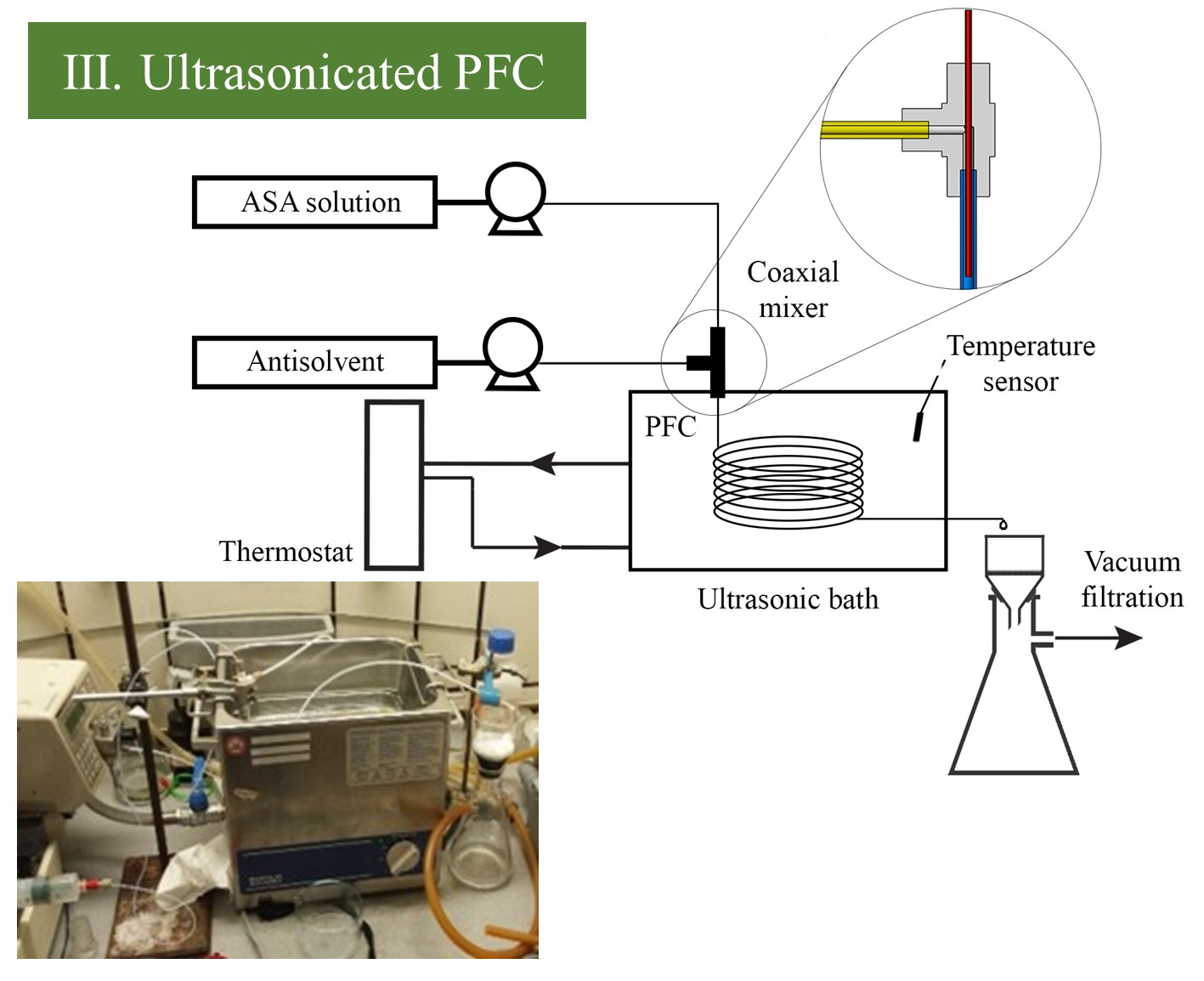
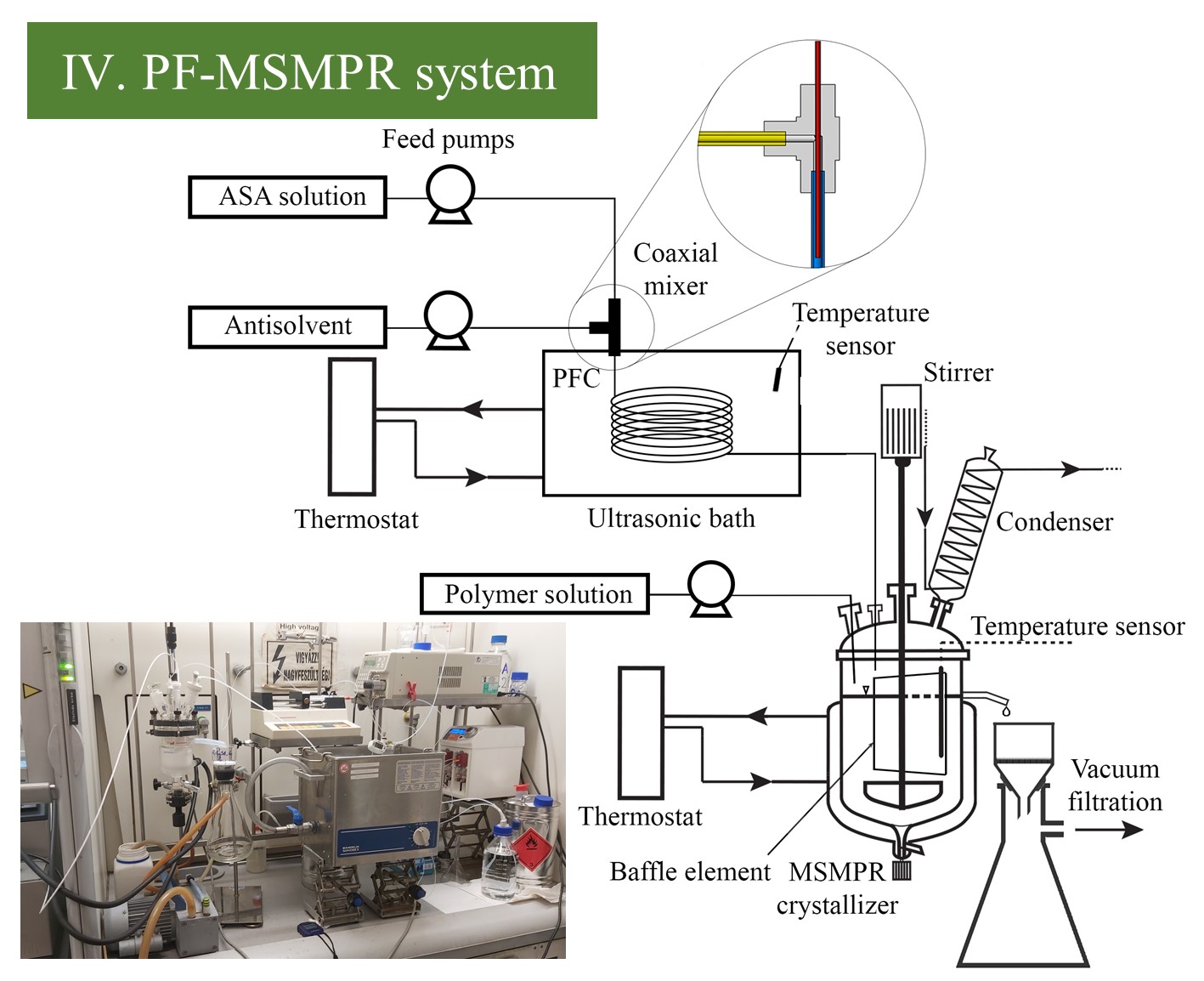
Figure 3 Schematic image and picture of PFC and PF-MSMPR system.
ASA solution and n-heptane antisolvent were fed into the crystallizers continuously with pumps, and the produced slurry was vacuum filtered directly after removal. The samples were dried at room temperature until constant weight.
The continuous experiments lasted for at least 15 residence times (up to 16 hours) to test the long-term operability of the systems. The product was sampled multiple times. A 22 or 23 Design of Experiment was performed to investigate the effect of crystallization parameters on product purity, crystal habit, crystal size, crystal size distribution, flowability, dissolution rate, yield, and productivity.
For the characterization of crystalline products, the following analytical techniques were used: HPLC, inverted microscope, crystal size distribution measurement with laser diffraction, chemical mapping based on Raman spectroscopy, flowability analysis, and dissolution test.
Results
The continuous crystallization of ASA from the synthesis mixture was fulfilled with high yield and purity. I found that the impurity content of the MSMPR and PF-MSMPR products was the lowest (0.3–0.4% on average), and it was slightly higher in the PFC products (0.7% on average). In the case of the PF-MSMPR system, the PVP-K30 additive content of the product was maximum of 7.7%, depending on the applied polymer amount.
The temperature and the antisolvent to ASA solution (AS/ASA) ratio significantly affected yield. Operated with similar process parameters, the TIJ-MSMPR, PFC, and PF-MSMPR systems performed similarly regarding yield (63–85%) and productivity (3.2–28.1 g/h depending on the process conditions).
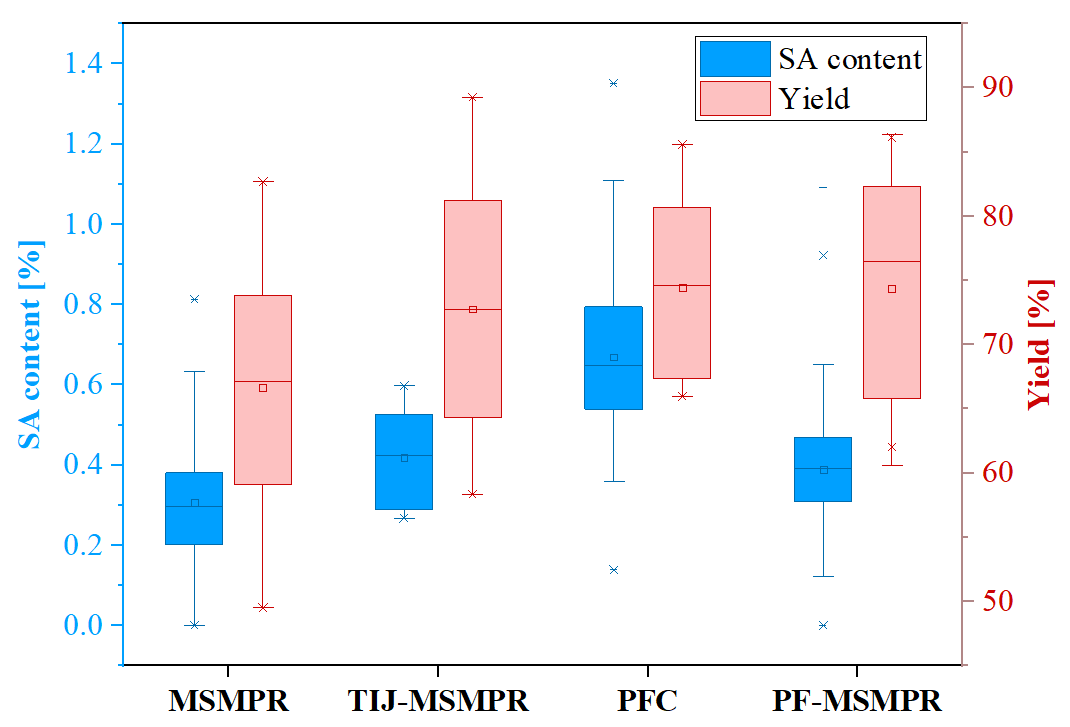
Figure 4 Comparison of the technologies based on SA impurity content and yield.
The technologies were significantly different regarding the onset of steady-state operation. In the case of the MSMPR crystallizer, the startup period lasted for hours (1.5–6 h), while PFC or PF-MSMPR product quality was invariable from the first sampling (PFC: 30–90 s, PF-MSMPR:8–25 min required).
The product of the MSMPR crystallizer consisted of large (~550 μm) isometric crystals and agglomerates with broad CSD. This product featured favorable flowability but slower dissolution (Figure 7), and it can potentially be further processed with direct compression. Smaller columnar, homogeneous crystals (<180 μm) were manufactured using a TIJ mixer. Small (<50 μm) needle-shaped crystals were formed with narrow CSD with PFC. This product has poor flowability properties; however, its dissolution is faster than the previous technologies so it can be a favorable method for active ingredients with slow dissolution.
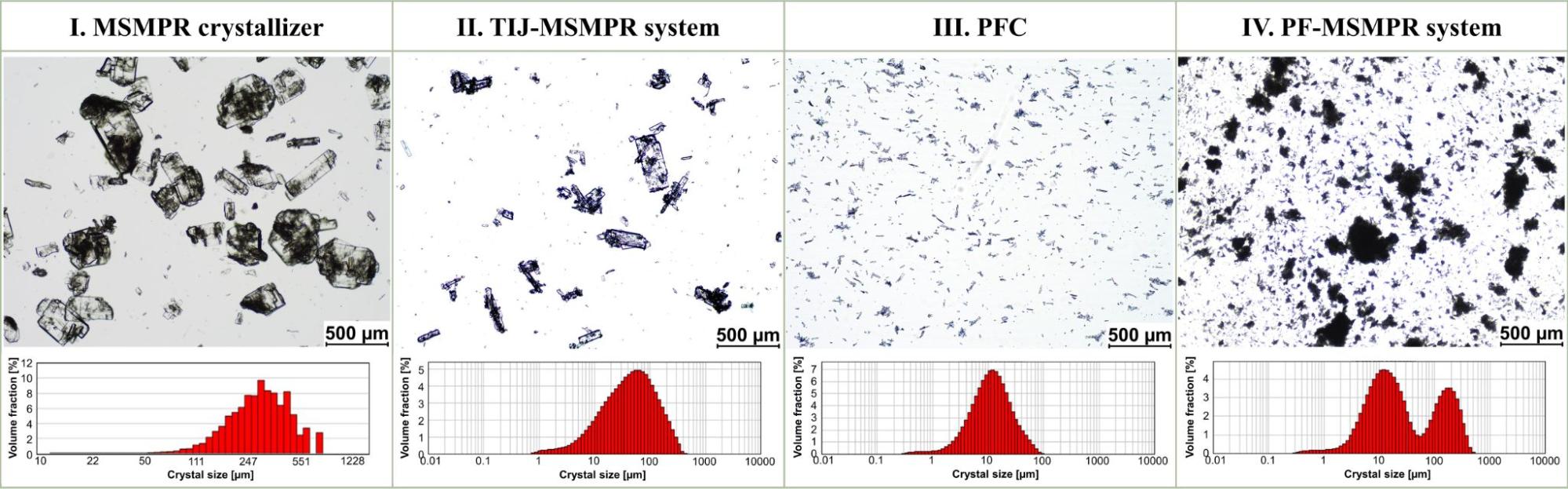
Figure 5 Microscopic pictures and CSD plots of the products (T:25°C).
The additive-assisted continuous crystallization in the connected PF-MSMPR system enables the combination of the advantages of MSMPR and PFC methods. Larger amounts (7.5–14%) of PVP-K30 promote the aggregation of primary particles produced in the PFC, which can result in aggregates up to 450 μm in size. Due to the morphological modification, the flowability of the product was improved by several categories. Namely, the flowability of the particles forming the aggregates reached the "fair" flowability class from the original "very poor" category.

Figure 6 Products with poor and favorable flowability properties.
However, as shown in Figure 7, the favorable dissolution properties of the PFC product that builds up the aggregates were retained.
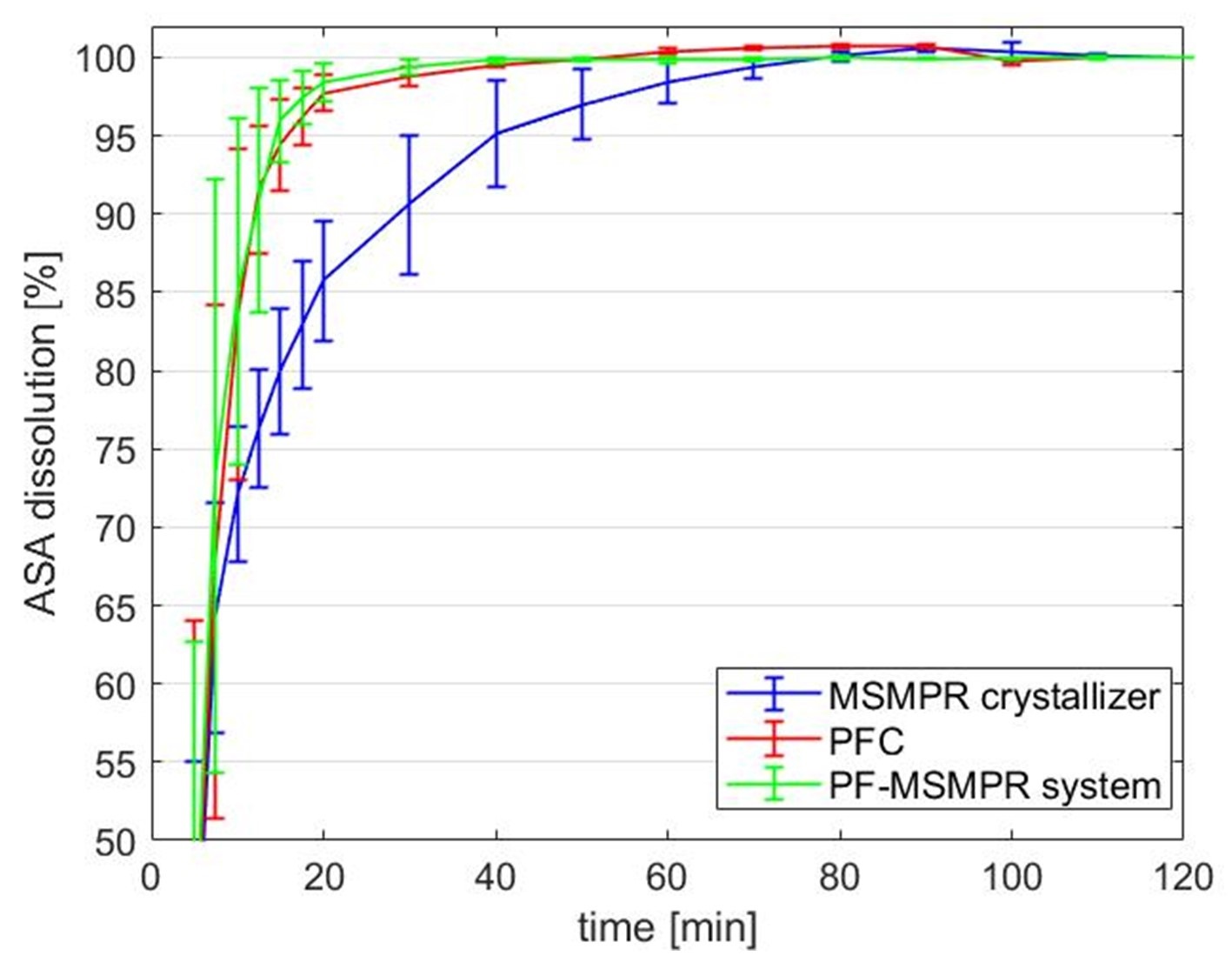
Figure 7 The dissolution rate of MSMPR, PFC, and PF-MSMPR products.
Expected impact and further research
I have developed various continuous crystallization technologies suitable for linking continuous synthesis and formulation procedures. I have characterized the process parameter dependence of product quality and quantity of the techniques in detail, revealing the product morphology modification potential of the alternatives. Thus, this study guides pharma researchers in choosing between these innovative continuous methods based on the technology features and the expected product characteristics. The research results illustrate the variety of strategies (crystallization process parameters, characteristics of the equipment used, method of mixing, use of additives) available for modifying the quality of the product; thus, it can also be a valuable tool for crystal engineering.
As a continuation of the research, my goal is to test the developed technologies for processing other synthesis mixtures (e.g., flibanserin) and to combine the technologies for further continuous processing steps (e.g., filtration, drying).
Publications, references, links
List of corresponding own publications (IF: impact factor, IC: independent citations)
[1] K. Tacsi, M. Gyürkés, I. Csontos, A. Farkas, E. Borbás, Z.K. Nagy, G. Marosi, H. Pataki, Polymorphic Concentration Control for Crystallization Using Raman and Attenuated Total Reflectance Ultraviolet-Visible Spectroscopy, Crystal Growth & Design, 2020, 20, 73–86.
IF: 4.076
[2] K. Tacsi, H. Pataki, A. Domokos, B. Nagy, I. Csontos, I. Markovits, F. Farkas, Z.K. Nagy, G. Marosi, Direct processing of a flow reaction mixture using continuous mixed suspension mixed product removal crystallizer, Cryst. Growth Des. 2020, 20, 4433–4442
IF: 4.076
[3] K. Tacsi, A. Joo, E. Pusztai, A. Domokos, Z.K. Nagy, G. Marosi, H. Pataki, Development of a triple impinging jet mixer for continuous antisolvent crystallization of acetylsalicylic acid reaction mixture, Chem. Eng. Process. Process Intensif. 2021, 165, 108446
IF: 4.237
[4] K. Tacsi, G. Stoffán, E. Pusztai, B. Nagy, A. Domokos, B. Szilágyi, Z.K. Nagy, G. Marosi, H. Pataki, Implementation of sonicated continuous plug flow crystallization technology for processing of acetylsalicylic acid reaction mixture, Powder Technology, 2022, 400, 117255
IF: 5.134
[5] A. Domokos, B. Nagy, M. Gyürkés, A. Farkas, K. Tacsi, H. Pataki, Y.C. Liu, A. Balogh, P. Firth, B. Szilágyi, G. Marosi, Z.K. Nagy, Z. K. Nagy, End-to-end continuous manufacturing of conventional compressed tablets: From flow synthesis to tableting through integrated crystallization and filtration, Int. J. Pharma., 2020, 581, 119297
IF: 5.875
[6] B. Nagy, B. Szilágyi, A. Domokos, K. Tacsi, H. Pataki, G. Marosi, Z. K. Nagy, Z.K. Nagy, Modeling of pharmaceutical filtration and continuous integrated crystallization-filtration processes, Chemical Engineering Journal, 2020, 413, 127566
IF: 13.273
[7] B. Nagy, B. Szilágyi, A. Domokos, B. Vészi, K. Tacsi, Z. Rapi, H. Pataki, G. Marosi, Z.K. Nagy, Z.K. Nagy, Dynamic flowsheet model development and digital design of continuous pharmaceutical manufacturing with dissolution modeling of the final product, Chem. Eng. J. 2021, 129947
IF: 13.273
[8] A. Domokos, L. Madarász, G. Stoffán, K. Tacsi, D. Galata, K. Csorba, P. Vass, Z.K. Nagy, H. Pataki. Real-Time Monitoring of Continuous Pharmaceutical Mixed Suspension Mixed Product Removal Crystallization Using Image Analysis, Organic Process Research & Development, 2022, 26, 1, 149–158
IF: 3.317
Table of links.
List of references.
[1] Mascia, S.; Heider, P. L.; Zhang, H.; Lakerveld, R.; Benyahia, B.; Barton, P. I.; Braatz, R. D.; Cooney, C. L.; Evans, J. M. B.; Jamison, T. F.; Jensen, K. F.; Myerson, A. S.; Trout, B. L. End-to-End Continuous Manufacturing of Pharmaceuticals: Integrated Synthesis, Purification, and Final Dosage Formation. Angew. Chem., Int. Ed. 2013, 52, 12359− 12363.
[2] Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895.
[3] M. Lin, Experimental and Numerical Study of Polymorphism in Crystallization Processes, 2020.
[4] Balogh, A.; Domokos, A.; Farkas, B.; Farkas, A.; Rapi, Z.; Kiss, D.; Nyiri, Z.; Eke, Z.; Szarka, G.; Örkényi, R.; Mátravölgyi, B.; Faigl, F.; Marosi, G.; Nagy, Z. K. Continuous End-to-End Production of Solid Drug Dosage Forms: Coupling Flow Synthesis and Formulation by Electrospinning. Chem. Eng. J. 2018, 350, 290–299.
[5] Domokos, A.; Nagy, B.; Gyürkés, M.; Farkas, A.; Tacsi, K.; Pataki, H.; Liu, Y. C.; Balogh, A.; Firth, P.; Szilágyi, B.; Marosi, G.; Nagy, Z. K.; Nagy, Z. K. End-to-End Continuous Manufacturing of Conventional Compressed Tablets: From Flow Synthesis to Tableting through Integrated Crystallization and Filtration. Int. J. Pharm. 2020, 581, 119297.
[6] Lührmann, M.-C.; Timmermann, J.; Schembecker, G.; Wohlgemuth, K. Enhanced Product Quality Control through Separation of Crystallization Phenomena in a Four-Stage MSMPR Cascade. Cryst. Growth Des. 2018, 18, 7323−7334
[7] Pal, S.; Madane, K.; Kulkarni, A. A. Antisolvent Based Precipitation: Batch, Capillary Flow Reactor, and Impinging Jet Reactor. Chem. Eng. J. 2019, 369, 1161−1171
