|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisor: Dr. Csontos István
Pharmaceutical applications of scaled-up electrospinning
Introducing the research area
The biopharmaceutical industry has seen incredible growth in recent decades. Electrospinning can be a breakthrough in the drying of bioactive ingredients, as it could replace the currently widespread freeze drying, which is an expensive batch process that often causes the degradation of the sensitive biopharmaceuticals. The advantage of electrospinning is its ability to provide nano- or microstructured solids by a gentle and continuous drying at room temperature. However, for electrospinning to be applied in the pharmaceutical industry the production capacities of the technology need to reach the commercial needs and it is essential to process the produced fibers into final dosage forms. Hence, my aim was to develop processable fibers and to produce fibers containing different types of biopharmaceuticals (proteins, whole cells) in a scaled up manner.
Brief introduction of the research place
The FirePharma research group is engaged in the development of innovative controlled, continuous and integrated pharmaceutical technologies in the pharmaceutical industry. Our group places great emphasis on building domestic and international industrial relations, and we currently maintain active cooperation with the majority of Hungarian as well as foreign pharmaceutical companies.
History and context of the research
The huge growth of biopharmaceuticals in the pharmaceutical industry is well illustrated by the fact that in the year of 2018, 14 of the 20 largest revenue-generating drugs in the world were biopharmaceuticals. The biopharmaceuticals market was valued at nearly $190 billion in 2017, which is projected to exceed $500 billion by 2025 [1].
The development of biopharmaceuticals is very challenging due to their low stability in aqueous media. Drying of these drugs can be a solution to stabilize an intermediate or final product, which generally improves stability and allows for easier and cheaper handling, transport and storage [2].
Currently, freeze drying is the most widely used technology to obtain solid biopharmaceutical formulations despite the disadvantages of the technology. Freeze drying is a highly energy- and time-intensive batch process that exposes biopharmaceuticals to a number of different stresses, which can result in degradation.
There is a growing interest in electrospinning, which is a new, efficient, and gentle continuous technology for solid biopharmaceutical production. Electrospinning provides instantaneous drying at room temperature, which is a great advantage for sensitive biopharmaceuticals. In addition, it is a simple, continuous and low-energy, and therefore cheap technology [4].
The majority of the publications related to electrospinning are focusing solely on how to manufacture fibers with appropriate characteristics, and only a few articles present ways to produce large amounts of fibers and convert the obtained fibers into applicable final dosage forms [5]. From the point of view of pharmaceutical application, it would be very important to investigate whether it is possible to produce processable biopharmaceutical-containing fibers in large quantities and whether it is possible to tablet the ground fibers. Investigating the impact of processing steps on the activity of sensitive biopharmaceuticals is also essential.
The research goals, open questions
During my research, I aimed to investigate the possibility of scaled-up electrospinning of aqueous solutions at room temperature by high-speed electrospinning using polymeric and polymer-free systems. My goal was also to develop processable biopharmaceutical-containing fibers and to produce oral solid formulations from the processed fibrous powders. In addition to studying the effect of processing steps on the biopharmaceuticals, I also aimed to investigate the long-term stability of the prepared solid formulations.
I worked with two different types of model biopharmaceuticals. One was β-galactosidase enzyme, a protein-type drug used to treat lactose intolerance, and the other was Clostridum butyricum, an important anaerobic bacterium from the gut microbiome. Clostridium butyricum produces butyric acid, which has been shown to have anti-inflammatory effects.
Methods
Clostridium butyricum was cultured at 37 °C under anaerobic conditions. After culturing, the cells were centrifuged. The resulting bacterial suspension or, in the other case, an aqueous solution of β-galactosidase was added to the polymer or cyclodextrin solutions from which the electrospinning was performed.
The scaled-up fiber production was carried out by high-speed electrospinning, a technology developed in our research group. Aqueous solutions of the biopharmaceuticals and the matrix were dispensed with a syringe pump into a stainless steel spinneret connected to high voltage. The high-speed rotation of the spinneret exerted centrifugal force on the solution, which got forced through the orifices of the spinneret and jet formation started immediately. The jets got thinner towards the ground into the nano or micro size range. Due to the increased specific surface area, the solvent evaporated extremely rapidly (<1 s). Solid fibers were collected either on a grounded collection plate or by special collection (e.g. cyclone).
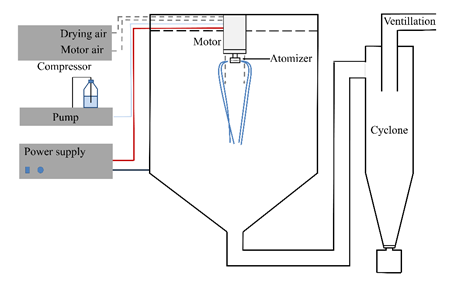

Figure 1 High-speed electrostatic fiberizing device (schematic diagram and photograph)
The processability of the fibers was investigated by grinding experiments. Three different methods were used: a sieve, a hammer mill, and an oscillating mill. The ground fibers were tableted after mixing with excipients. The long-term stability of biopharmaceuticals is essential for their use in the pharmaceutical industry, so it is important to investigate whether the developed new formulations are suitable for maintaining the storage stability of biopharmaceuticals. Samples were stored in a closed sample holder during the long-term stability test (6–12 months).
The viability of Clostridium butyricum in the fibers and in the prepared tablets was examined by measuring the colony-forming units and the enzymatic activity of β-galactosidase was determined by monitoring an enzyme-catalyzed reaction.
For the physicochemical characterization of the fibrous materials and the prepared tablets the following analytical techniques were used: scanning electron microscopy, thermogravimetry, modulated differential scanning calorimetry, powder X-ray diffraction, chemical mapping based on Raman spectrometry, fracture strength measurement.
Results
I successfully realized the scaled-up production of enzyme- and bacteria-containing fibers at room temperature using high-speed electrospinning. The maximum productivity achieved was 270 g/h, which is 300 x higher than what can be achieved with traditional fiberization methods.

Figure 2 Scaled-up production of biopharmaceuticals-containing fibers
The produced fibers had poor flowability properties, and thus it was necessary to grind the fibers to be suitable for the final dosage form (in this case a tablet). The fibers were easily grindable even without secondary drying. Scanning electron microscopy images showed that the fibers were broken into shorter fragments but retained their fibrous structure.
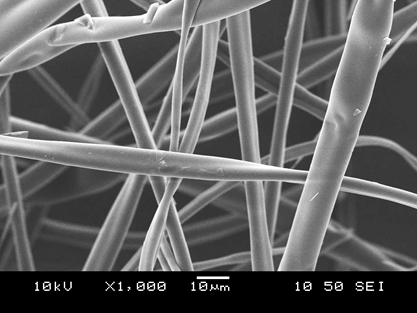

Figure 3 Scanning electron microscopy images of bioactive fibers before and after milling
After mixing the milled fibers with tableting excipients, the resulting powders already had adequate flowability and were thus suitable for tableting by direct compression.

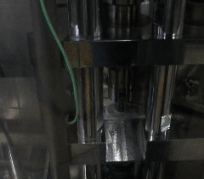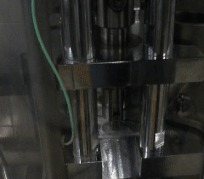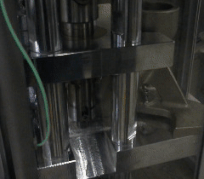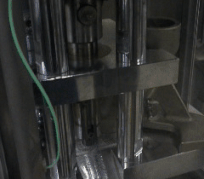
Figure 4 Automatic tableting of ground biopharmaceutical-containing fibers
I investigated the effect of manufacturing and processing steps (fiber formation, milling, tableting) and long-term storage on enzyme activity and bacterial cell viability.
For β-galactosidase, no significant decrease in activity was observed after any of the processing steps. In addition, the enzyme remained stable in the tablets after 1 year of storage at both 4 °C and room temperature.
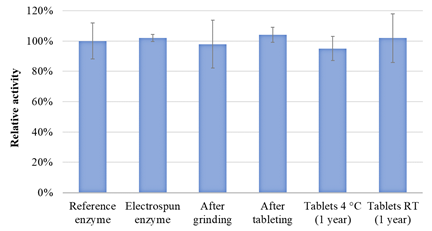
Figure 5 β-galactosidase activity after electrospinning, milling, tableting, and 1-year storage of tablets (storage at 4 °C and room temperature)
Bacterial viability was slightly reduced after mixing into the electrospinning solution and fiber formation. The decrease in viability may be attributed to the sudden change in the osmotic environment of vegetative cells and spores upon mixing into solution or exposure to high voltage and instantaneous solvent evaporation during fiber formation. The bacteria have retained their ability to produce butyric acid in the fibers, indicating that this new solid form has real therapeutic potential. By measuring the amount of colony forming units, I proved that the processing steps (grinding, tableting) do not significantly reduce the number of viable bacteria. In general, the results of the storage test showed that samples stored in an anaerobic environment had better survival than samples stored in the presence of oxygen at the same storage temperature. However, samples stored in the presence of oxygen at room temperature still contained enough living cells to be used in a pharmaceutical formulation.
In summary, the gentle drying achieved by high-speed electrospinning and the processable matrices made it possible to produce final oral dosage forms from the studied model biopharmaceuticals without significant decrease in activity.
Expected impact and further research
I have shown that high-speed electrospinning is a promising technology for the gentle drying of biopharmaceuticals, which is able to eliminate the disadvantages of freeze-drying. A significant part of this work was carried out in collaboration with Janssen scientists (Pharmaceutical Companies of Johnson & Johnson). Therefore, the obtained results can generate substantial industrial interest and the utilization of these results can certainly be foreseen in the future.
As a continuation of the research, I investigate the aseptic production possibilities of fibers, as in the case of parenteral final dosage forms, the sterility of the solid form is crucial.
Publications, references, links
List of corresponding own publications (IF: impact factor, IC: independent citations)
[1] P. Vass, B. Démuth, E. Hirsch, B. Nagy, S.K. Andersen, T. Vigh, G. Verreck, I. Csontos, Z.K. Nagy, G. Marosi, Drying technology strategies for colon-targeted oral delivery of biopharmaceuticals, Journal of Controlled Release, 296 (2019) 162-178.
IF: 7.877 IC: 18
[2] P. Vass, B. Démuth, A. Farkas, E. Hirsch, E. Szabó, B. Nagy, S.K. Andersen, T. Vigh, G. Verreck, I. Csontos, G. Marosi, Z.K. Nagy, Continuous alternative to freeze drying: Manufacturing of cyclodextrin-based reconstitution powder from aqueous solution using scaled-up electrospinning, Journal of Controlled Release, 298 (2019) 120-127.
IF: 7.877 IC: 15
[3] P. Vass, E. Szabó, A. Domokos, E. Hirsch, D. Galata, B. Farkas, B. Démuth, S.K. Andersen, T. Vigh, G. Verreck, G. Marosi, Z.K. Nagy, Scale‐up of electrospinning technology: Applications in the pharmaceutical industry, WIREs Nanomedicine and Nanobiotechnology, (2019) e1611.
IF: 6.140 IC: 7
[4] P. Vass, E. Hirsch, R. Kóczián, B. Démuth, A. Farkas, C. Fehér, E. Szabó, Á. Németh, S.K. Andersen, T. Vigh, G. Verreck, I. Csontos, G. Marosi, Z.K. Nagy, Scaled-up production and tableting of grindable electrospun fibers containing a protein-type drug, Pharmaceutics, 11 (2019) 329.
IF: 4.773 IC: 2
[5] P. Vass, Z.K. Nagy, R. Kóczián, C. Fehér, B. Démuth, E. Szabó, S.K. Andersen, T. Vigh, G. Verreck, I. Csontos, G. Marosi, E. Hirsch, Continuous drying of a protein-type drug using scaled-up fiber formation with HP-β-CD matrix resulting in a directly compressible powder for tableting, European Journal of Pharmaceutical Sciences, 141 (2020) 105089.
IF: 3.532 IC: 0
[6] P. Vass, E. Pantea, A. Domokos, E. Hirsch, Á. Németh, M. Molnár, Cs. Fehér, S.K. Andersen, T. Vigh, G. Verreck, I. Csontos, G. Marosi, Z.K. Nagy, Electrospun Solid Formulation of Anaerobic Gut Microbiome Bacteria, AAPS PharmSciTech, (2020).
IF: 2.401 IC: 0
[7] K. Kiss, P. Vass, A. Farkas, E. Hirsch, E. Szabó, G. Mező, Z.K. Nagy, G. Marosi, A solid doxycycline HP-β-CD formulation for reconstitution (i.v. bolus) prepared by scaled-up electrospinning, Int. J. Pharm., 586 (2020) 119539.
IF: 4.845 IC: 0
[8] E. Szabó, B. Démuth, D.L. Galata, P. Vass, E. Hirsch, I. Csontos, G. Marosi, Z.K. Nagy, Continuous formulation approaches of amorphous solid dispersions: Significance of powder flow properties and feeding performance, Pharmaceutics, 11 (2019) 654.
IF: 4.773 IC: 1
[9] E. Hirsch, P. Vass, B. Demuth, Z. Petho, E. Bitay, S.K. Andersen, T. Vigh, G. Verreck, K. Molnar, Z.K. Nagy, G. Marosi, Electrospinning scale-up and formulation development of PVA nanofibers aiming oral delivery of biopharmaceuticals, Express Polymer Letters, 13 (2019) 590-603.
IF: 2.875 IC: 4
[10] I. Wagner, Z.K. Nagy, P. Vass, C. Fehér, Z. Barta, T. Vigh, P.L. Sóti, A.H. Harasztos, H. Pataki, A. Balogh, G. Verreck, I.V. Assche, G. Marosi, Stable formulation of protein-type drug in electrospun polymeric fiber followed by tableting and scaling-up experiments, Polymers for Advanced Technologies, 26 (2015) 1461-1467.
IF: 1.823 IC: 5
Links
List of references
[1] G. Walsh, Biopharmaceutical benchmarks 2018, Nat. Biotechnol., 36 (2018) 1136-1145.
[2] A. Langford, B. Bhatnagar, R. Walters, S. Tchessalov, S. Ohtake, Drying technologies for biopharmaceutical applications: Recent developments and future direction, Drying Technol., (2017) 1-8.
[3] W. Wang, Lyophilization and development of solid protein pharmaceuticals, Int. J. Pharm., 203 (2000) 1-60.
[4] P. Mehta, R. Haj-Ahmad, M. Rasekh, M.S. Arshad, A. Smith, S.M. van der Merwe, X. Li, M.-W. Chang, Z. Ahmad, Pharmaceutical and biomaterial engineering via electrohydrodynamic atomization technologies, Drug Discov. Today, 22 (2017) 157-165.
[5] B. Démuth, A. Farkas, B. Szabó, A. Balogh, B. Nagy, E. Vágó, T. Vigh, A.P. Tinke, Z. Kazsu, Á. Demeter, J. Bertels, J. Mensch, A. Van Dijck, G. Verreck, I. Van Assche, G. Marosi, Z.K. Nagy, Development and tableting of directly compressible powder from electrospun nanofibrous amorphous solid dispersion, Adv. Powder Technol., 28 (2017) 1554-1563.
