|
|
BMe Research Grant |
|
Doctoral School of Psychology/Cognitive Sciences
MTA TTK Neuronal Networks and Behaviour Research Group
Supervisor: Dr. Mátyás Ferenc
Controlling arousal
Introducing the research area
Controlling arousal and the sleep-wake cycle is one of the most fundamental elements of behaviour. An under-aroused brain can lead to boredom, loss of attention, sleepiness or even coma. On the other hand, an overstimulated central nervous system can trigger stress, anxiety and panic. In some cases it is important to enter these brain states, but in other situations it can be dangerous or counterproductive. For example, both calmness and light stress can contribute to successfully pass an exam. However, a well-prepared, but overstressed or a sleepy student is more likely to fail the same test1. Not to mention that, occurrence of sleep-related disorders, such as insomnia is increasing in the developed world. Therefore, it is very important to understand the brain system that controls arousal.
Brief introduction of the research place
The Neuronal Network and Behaviour Research group of the MTA TTK was founded in 2015 by Dr. Ferenc Mátyás. The group’s main focus is the cell-specific investigation of different brain processes, such as sleep and emotional behaviour. In our experiments we combine viral-based neuroanatomical, multisite in vivo electrophysiological and behavioural approaches in special transgenic animal models (mouse).
History and context of the research
It was suggested several decades ago that the medial part of the so called thalamus (an ancient structure in the middle of the brain) is essential in the control of arousal-related neural processes2. The first experiments investigating this theory demonstrated that electric stimulation of the medial thalamus led to desynchronization of cortical activity, which is the electrophysiological sign of wakefulness3. In accordance with this, deep brain stimulation of this brain region improved motor and cognitive skill in some comatose patients4 (Figure 1).
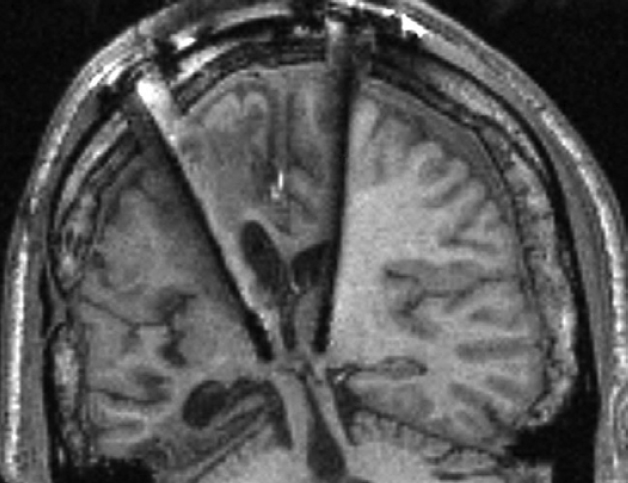
Figure 1. Deep brain stimulation of the MT of a comatose patient. The stimulators can be seen in the middle of the image. The patient’s motor and cognitive skill temporarily improved following the stimulation. Source: N. D. Schiff et al. 2007
However, electric stimulation is a non-specific experimental approach. It means that using this method, one cannot be sure about the source of the behavioural change (i.e. waking up), because the electricity provided by the stimulation probably reaches several cell-types, or even completely different brain regions (Figure 2). This can be the reason why the efficacy of these treatments is relatively low, and thus, these are still not common in clinical use. Similar pitfalls are also present in other widely used neuroscientific methods, such as lesions and pharmacological activation/inactivation. Using these techniques does not reveal either which neurons of the thalamus are responsible for this arousal-promoting feature. Therefore, our research group combined several cutting edge experimental tools, such as special genetically modified experimental animal models and altered virus strains in order to reveal the exact source of this thalamic arousal regulation.
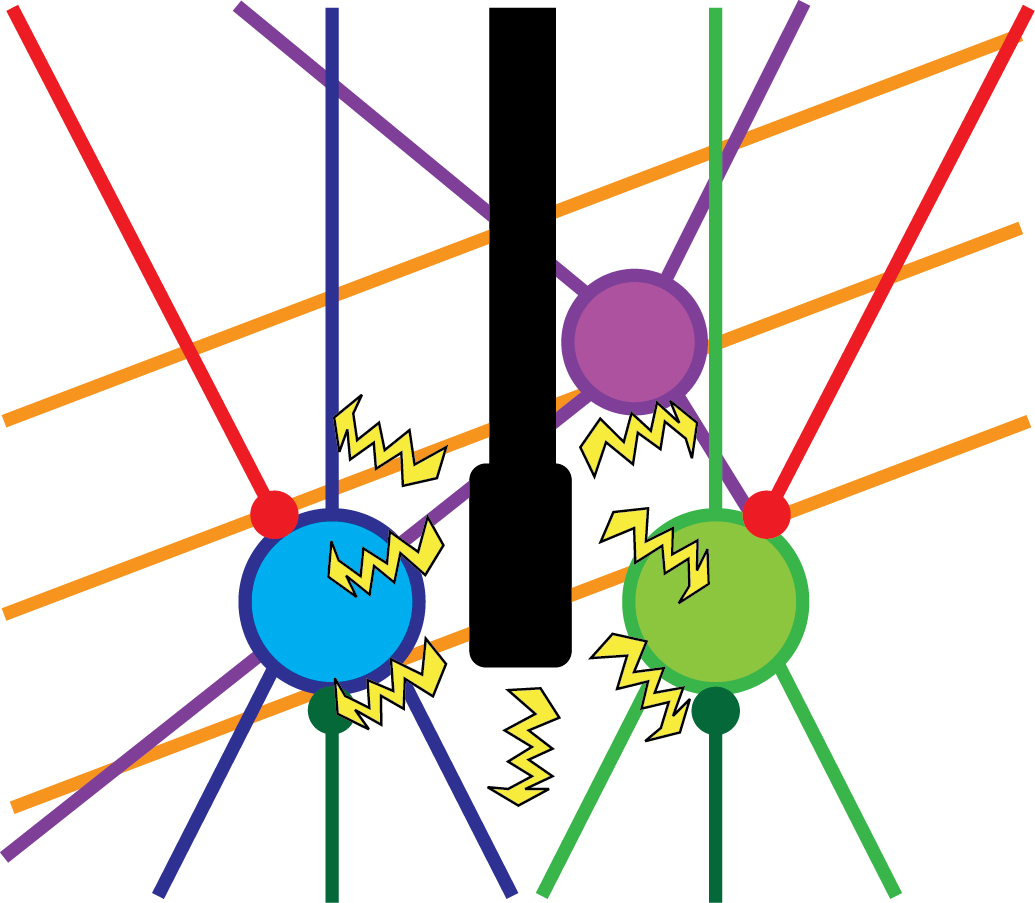
Figure 2. Non-specific experimental approaches. A brain region can contain several different cell-types (blue, green, purple), such as inhibitory or excitatory cells. Besides, axons from different brain regions can innervate the given brain region (red and dark green lines), not to mention axons that run through the given region (orange lines). Electrical stimulation can affect all these elements, therefore the source of the behavioural change cannot be identified. Own figure.
The research goals, open questions
Previous experiments demonstrated that the medial part of the thalamus (MT) is connected to a network involved in arousal related processes. These regions include the prefrontal cortex (PFc, involved in consciousness and emotion control), the amygdala (Amy, emotional learning) and the nucleus accumbens (NAc, reward and motivation), among others5-7. Our main goal was to identify a genetic marker that is specifically expressed in the neurons of the MT that innervate these brain regions. Without such a marker, it is not possible to specifically investigate MT nuclei (especially in mice), due to their irregular shape, small size and localisation among functionally different brain regions. By identifying such a marker, we would be able to specifically reveal the anatomical, electrophysiological and behavioural characteristics of these cells and the related brain circuits.
Methods
First, we used classical track tracing tools to identify the source of innervation of the above mentioned brain regions (PFc, Amy, NAc). In these experiments, we injected small amounts of neuronal tracers into these regions using small glass pipettes in living animals (Figure 3). After one week, we could harvest the brain tissue and look for labelled neurons. Interestingly, almost all neurons in the MT projecting to these brain areas were localised among cells expressing a special calcium-binding protein, calretinin (CR). Using high magnification microscopy, we found out that the vast majority (~95%) of PFc-, Amy- and NAc-projecting MT cells actually expressed CR. We also revealed that these CR-expressing MT cells specifically activated during wakefulness, and other arousing (stressful) situations (e.g. handling of the animals, mild electric shock). In other words, CR was identified as a specific molecular/genetic marker for the arousal sensitive MT cells (Figure 4).
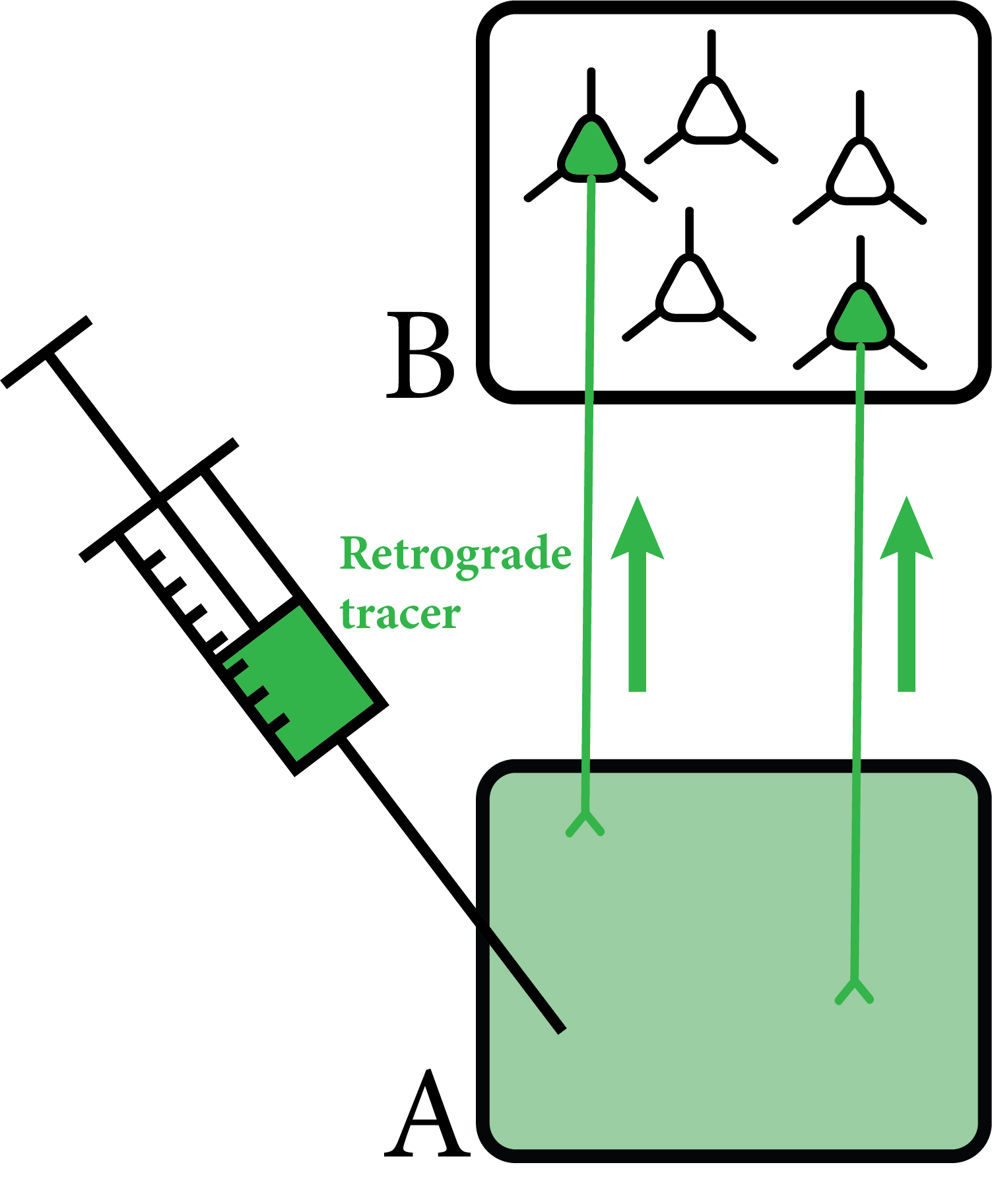
Figure 3. Retrograde tracing. In these experiments the tracer (green) is injected to the given brain region (A). Neurons projecting to this area absorb the tracer, and transport it (arrows) to their cells bodies in another brain region (B). Therefore, source of the innervation can be revealed. Own figure.
Having this marker in hand, we could turn to cell-specific approaches. First, we obtained a special mouse strain, the so called calretinin-Cre (CR-Cre) strain. In these animals all CR-expressing cells (and only these cells) express another protein that works as “molecular scissors”. These scissors don’t influence normal neuronal operation, but can “cut” external and non-functioning DNA strands in specific locations to turn it into functional ones.
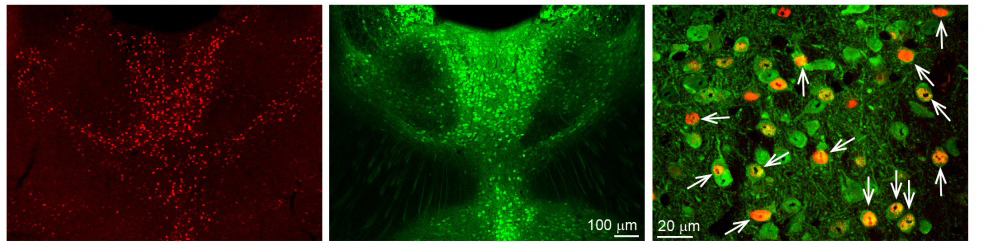
Figure 4. Calretinin (CR) cells are selectively activated during arousal. With a specific gene (cFos) activation of neurons can be traced. cFos-expressing cells (activated by arousal) in the MT can be seen in red (first panel). Calretinin (green) is expressed in a highly overlapping manner with these activated cells (second panel). In the higher magnification picture (third panel) the overlap of the two populations can be seen (arrows). Source: MTA KOKI
Besides these mice, we needed special viruses that carry necessary genes (for
example genes of fluorescent proteins) in their viral DNA. If we inject these
viruses in the brains of these CR-Cre animals, the virus only infects cells
that express the “molecular scissors” (Cre). In these cells the gene carried
by the viral vector can be expressed, while all other cells are unaltered. See
Figure 5 for the summary of this method.
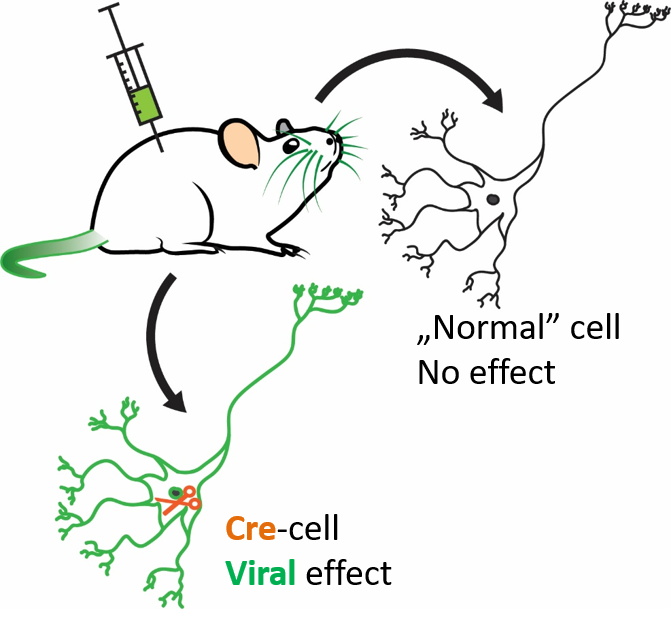
Figure 5. Cell-specific approach. In special (genetically modified) mouse strains, given cells (e.g. calretinin-expressing cells) express the Cre protein (orange), which acts as “molecular scissors”. Injecting special viruses to these animals lead to the expression of the protein carried by the virus (green) in the Cre-containing (e.g. calretinin-expressing) cells. Every other cell is unaffected by the virus. Own figure.
Using these tools, first we injected a virus that carried the genes of fluorescent (“luminous”) proteins into the MT of CR-Cre mice. This way, we selectively labelled the CR-expressing MT cells and so their axons (long branches of neurons that innervate other brain regions). After this we could search for the axons of MT cells throughout the entire brain.
Besides the anatomical experiments, we also carried out behavioural investigations in the same mouse strain. In this case, first we injected a similar viral vector to the MT, but this time it carried the gene of a light-sensitive ion-channel. These channels let electrically charged ions in and out of the cell. This ion-flux constitutes the basis of electric cellular communication. Light-sensitive ion-channels are special, because they only open if a specified wavelength light reaches them. So, we implanted tiny optical fibres in the MT of the same mice, so we could illuminate (activate or deactivate) these cells during relevant arousal states (e.g. sleep) and monitor the change of behaviour. This technique is called specific optogenetics. Thus, using the proper virus vectors and light sources, cells can be activated and deactivated with high spatial and temporal precision (Figure 6).
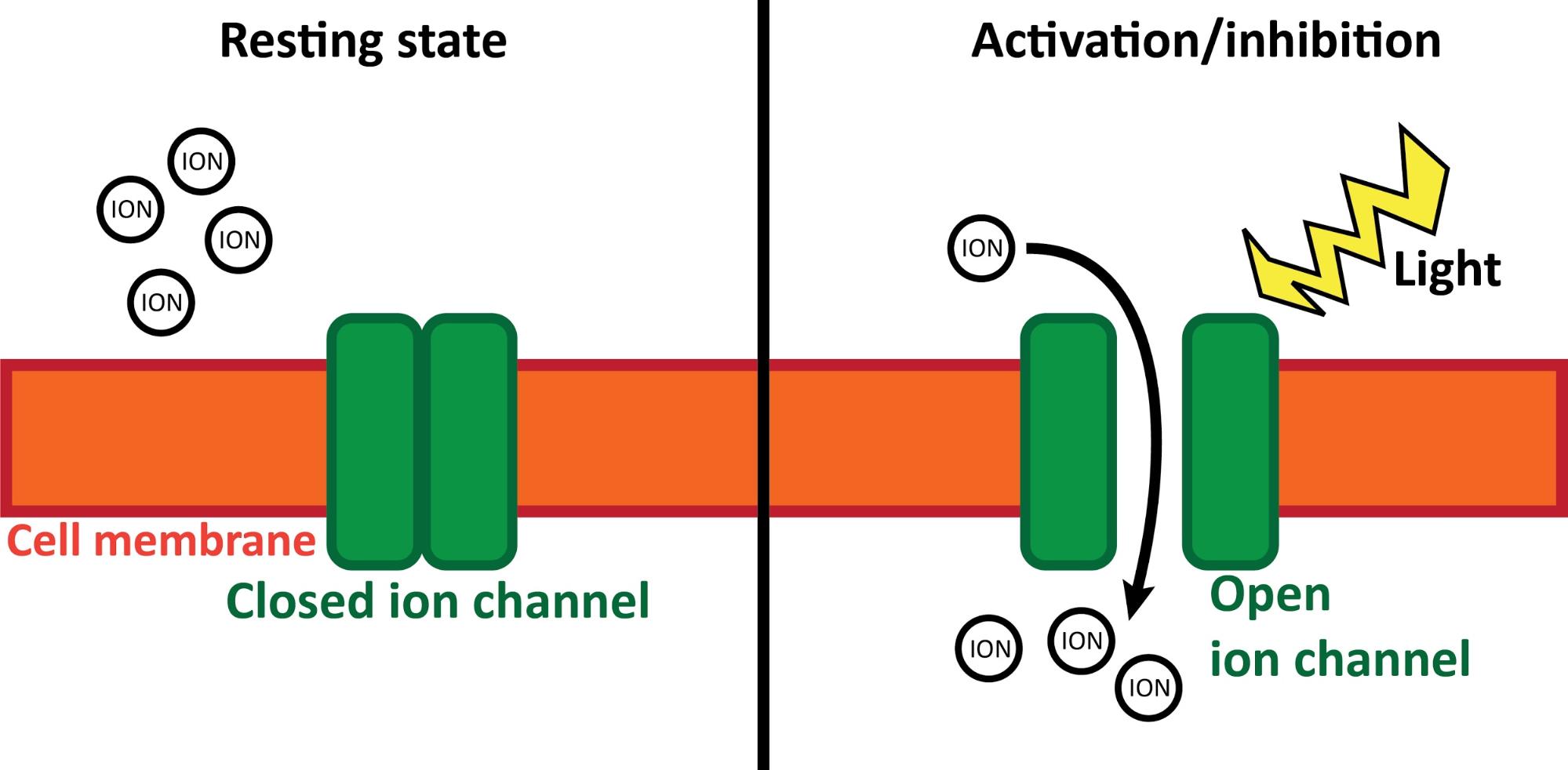
Figure 6. Optogenetics. During inactive (resting) state (left) light-sensitive ion channels (green) are closed in the cell membrane (orange). They do not affect normal cell processes. If a specific wavelength light (yellow) illuminates the ion channels (right), they open and let ions (charged particles) in or out of the cell. This leads to the activation/inhibition of the cell (depending on the exact type of the ion channel). Own figure.
Results
Specific anatomical experiments revealed the projection-pattern of CR-expressing MT cells. Axons of these cells were found in the PFc, Amy and NAc, and also other brain regions known to be innervated by MT. Considering that the regions innervated by the MT all participate in behaviourally and emotionally relevant processes (together forming the so called limbic system), and that MT can simultaneously influence their activity, MT is an ideal candidate for a central arousal-controlling hub.
To investigate this, we used specific optogenetic approach. First, animals were tested during a natural exploratory behaviour. We decreased the activity of CR-expressing MT cells during this exploratory phase using the above described optogenetic approach. Compared to the control group, animals in the test group showed lower level of locomotor activity. This can be explained by decreased levels of arousal, caused by the silenced MT cells. In other words, decreased activity of CR-expressing cells of the MT cause decreased levels of arousal in naturally behaving mice.
We also explored the effect of MT stimulation in the sleep-wake cycle. With the same optogenetic approach, CR-expressing MT cells were selectively activated in naturally sleeping animals. Strong stimulation of these cells led to the awakening of these animals, while animals of the control group showed no sign of elevated arousal. It is important to note that the awakening of the animals mimicked a completely natural awakening, without any sign of distress, pain or fear (Figure 7).
Weaker stimulations in the same experimental condition caused short awakening, called microarousal. During these, animals showed only small head and neck movements, and then returned to normal sleep.
Taken together, our data demonstrate that CR-expressing MT cells are selectively activated during arousing situations and their axons innervate several cortical and subcortical brain regions involved in different behaviourally and emotionally relevant processes. Therefore, these cells are in an ideal position to serve as a central arousal controlling hub of the brain. In accordance with this, our behavioural experiments confirmed that silencing of these cells lead to decreased levels of arousal, while activation of the same cells trigger awakening in a “dose-dependent” manner (i.e. stronger activation - higher arousal).
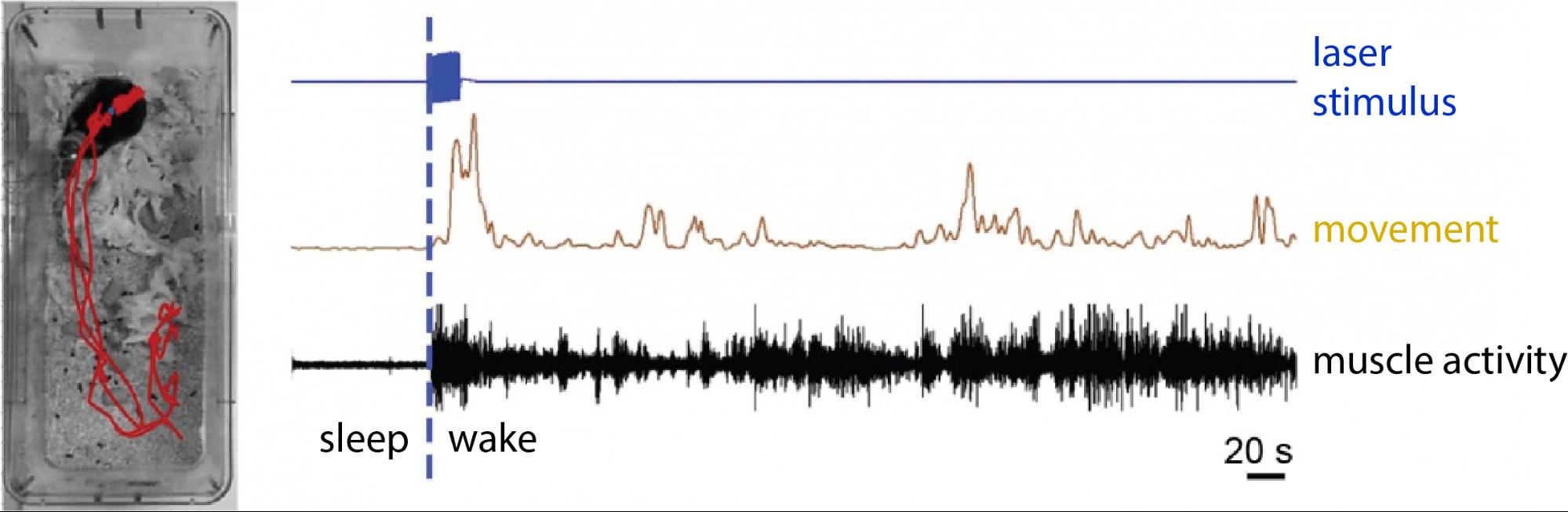
Figure 7. Awakening elicited by the activation of MT cells. In the picture on the left the animals movement-track can be seen (red) following the specific optogenetic activation of CR-expressing MT cells. The animal was sleeping before the activation. On the right the movement record (brown) and the elevated muscle activity (black) are seen following the laser stimulus (blue). Source: MTA KOKI
Expected impact and further research
We described a previously unidentified cell population in the MT, the CR-expressing cells that can selectively control arousal. Since these cells are also present in the human thalamus with similar connectivity patterns, our results can help understand the human sleep-wake cycle better. With a genetic marker in hand, it could be possible to develop new kinds of sleeping medications that can mimic natural sleep better than the currently available sedatives. Further research should be focusing on the more detailed description of these cells to find specific receptors or other specific molecular characteristics that can act as a pharmaceutical target.
Publications, references, links
Scientific publications:
A highly collateralized thalamic cell type with arousal-predicting activity serves as a key hub for graded state transitions in the forebrain
Mátyás, F. ; Komlósi, G. ; Babiczky, Á. ; Kocsis, K. ; Barthó, P. ; Barsy, B. ; Dávid, C. ; Kanti, V. ; Porrero, C. ; Magyar, A. ; Szűcs, I. ; Clasca, F. ; Acsády, L
NATURE NEUROSCIENCE 21 : 11 pp. 1551-1562. , 12 p. (2018)
DOI: https://doi.org/10.1038/s41593-018-0251-9
Plastic multimodal signaling of a thalamo-amygdala pathway controls associative behavior
Boglárka Barsy; Kinga Kocsis; Aletta Magyar; Ákos Babiczky; Mónika Szabó; Judit M. Veres; Daniel Hillier; István Ulbert; Ofer Yizhar; Ferenc Mátyás (in prep.)
Other publications:
Függőségi
helyzetjelentés – Interjú Demetrovics Zsolt addiktológussal
National Geographic Magyarország Online,
2019.06.04.
Három tévhit
agyunk működéséről
National Geographic Magyarország Online,
2019.03.25.
Évek óta
dolgozom kísérleti egerekkel. De mit szólnak ehhez az állatvédők?
Qubit.hu,
2019.03.01.
Górcső alatt az
állatkísérletek: tudományos érvek és állatvédelem
Tudományos eredmények a nagyvilágból – Válogatás a Campus Mundi ösztöndíjasok
tanulmányaiból (2018)
Sírás és
nevetés, avagy a félelmi- és a jutalmazási rendszer az agyban 3/3
National Geographic Magyarország Online,
2018.12.30.
Jutalmazás,
dopamin és az agy - Félelem és jutalmazás az agyban 2/3
National Geographic Magyarország Online,
2018.09.20.
Hogyan fél az agyunk? Félelem és jutalmazás az agyban 1/3
National Geographic Magyarország Online, 2018.05.24.
Table of links:
Index.hu report (in Hungarian)
List of references:
1. R. M. Yerkes, J. D. Dodson, The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 18, 459–482 (1908).
2. Y. D. Van der Werf, M. P. Witter, H. J. Groenewegen, The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 39, 107–40 (2002).
3. R. Morison, E. Dempsey, A study of thalamo-cortical relations. Am. J.Physiol. 135, 281–292 (1941).
4. N. D. Schiff et al., Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 448, 600–3 (2007).
5. R. P. Vertes, W. B. Hoover, Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J. Comp. Neurol. 508, 212–37 (2008).
6. W. B. Hoover, R. P. Vertes, Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct. Funct. 217, 191–209 (2012).
7. R. P. Vertes, S. B. Linley, W. B. Hoover, Limbic circuitry of the midline thalamus.
Neurosci. Biobehav. Rev. (2015), doi:10.1016/j.neubiorev.2015.01.014.
