|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Physical Chemistry and Materials Science / Surface Chemistry Group
Supervisor: Dr. László Krisztina
Retard Drug Release with Intelligent Polymer Gel
Introducing the research area
Biocompatible polymergel drug carriers have several properties similar to those of human tissues, such as soft structure, high water content and the ability to respond to environmental stimuli (Fig.1/a). Contrary to conventional formulation, responsive gels may open new perspectives in drug release by controlling its conditions (dose, speed, time, site), thus minimizing side-effects and the amount of drug used. The subject of my investigations was the release mechanism determining interactions between drug molecules and their carrier on one of the most studied temperature-responsive polymers, the poly(N-isopropylacrylamide) (PNIPA) (Fig.1/b).
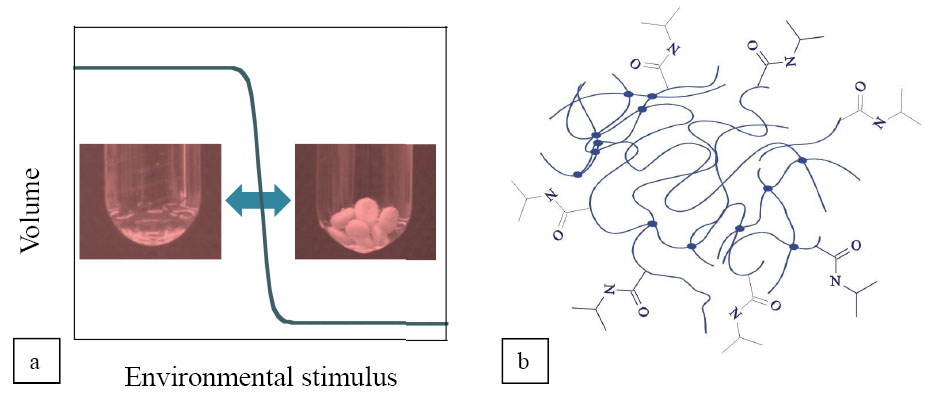
Fig.1 Drug uptake and release of PNIPA can be controlled by several environmental parameters (e.g. temperature, solution concentration, ionic strength)
Brief introduction of the research place
The Surface Chemistry Group of the Department of Physical Chemistry and Materials Science focuses on the preparation, surface phenomena and application of different materials with tuned porosity and surface chemistry, in extensive Hungarian and international collaborations. The range of investigated materials includes conventional activated carbon; carbon nanoparticles with unique physico-chemical properties (e.g. carbon nanotube, graphene); extremely low density carbon aerogels; metal organic compounds for gas storage applications; soft polymer gels and coupled systems.
History and context of research
Responsive hydrogels are three-dimensional, cross-linked polymers that can absorb large quantities of water. In case of PNIPA this amount of water can be 30-40 times the weight of the gel. Its ability to uptake, store and release drug molecules may make PNIPA an outstanding vehicle for targeted and controlled drug delivery. PNIPA is intensively studied because it exhibits a non-linear volume phase transition close to the natural temperature of the human body (34 °C). The inflammations related temperature increase can induce drug release. Temperature responsive gels shrink upon temperature elevation resulting in a sudden increase of the concentration of the drug that is discharged with the swelling liquid. Contrary to this prompt release, a new research direction can be the retard type drug release based on the shape memory of the gel carrier (Fig.2).

Fig.2 The gel carrier can be easily loaded with drug then stored in dry state. The release of drug molecules is determined by the rate of reswelling.
The research goal, open questions
The disadvantage of drug transport systems based on the quick phase transition of swollen gels is that they are not suitable for a retard drug release. A possible solution can be the application of dry drug loaded gels that provide prolonged release during their slow reswelling (Fig.3).
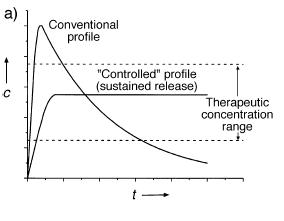
Fig.3 Drug concentration changes in conventional and prolonged release
The effectiveness of drug release is determined by several factors, and among them drug–polymer interactions are of vital importance. These not only influence the amount of drug that can be released but also their morphology (amorphous/crystalline, crystallite size, etc.), too. Drug - polymer interactions may significantly influence the effectiveness of the formula. By drying the loaded gel matrices, amorphous or small crystalline particles can develop from the drug that facilitate accurate dosage, dissolution and thus bioavailability. Despite numerous studies on PNIPA gels in the past decades, its interactions with guest molecules are not fully explored. The goal of my research was to obtain a deeper insight into the interactions between PNIPA and some drug molecules both at macroscopic and molecular levels.
Methods
Phenol (Fig.4/a) is widely used as a model of several aromatic drugs. In my research, first the effect of OH-substituted phenols on the transition of PNIPA was investigated. Molecules with phenolic hydroxyl groups can only show a slight effect or no effect at all on the properties of PNIPA, while others can change the conditions of phase transition even at low additive concentrations [1-4]. Thereafter, the effects of two aromatic drugs that were expected to behave differently were compared: dopamine (4-(2-aminoethyl)benzene-1,2-diol) (Fig.4/b) and ibuprofen (2-(4-isobutylphenyl)-propionic acid) (Fig.4/c). Dopamine acts as a hormone and neurotransmitter in the human brain and nervous system. Abnormal levels of this molecule may result in Parkinson's disease. Ibuprofen is an analgesic, antipyretic and non-steroidal antiimflammatory drug (NSAID).Drug molecules were applied in their salt forms in accordance with pharmaceutical practice.
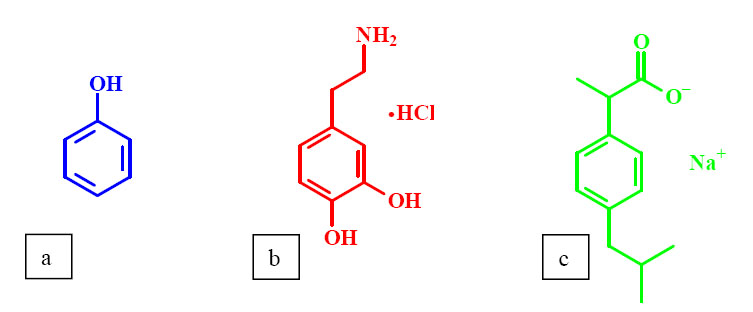
Fig.4 Structure of the PNIPA hydrogel and the model drug molecules: (a) PNIPA (b) phenol (c) dopamine hydrochloride (d) ibuprofen sodium
The mechanical properties of the gels were characterized by their Young's moduli. Drug loaded PNIPA gels were investigated by swelling experiments [EM1] and differential scanning microcalorimetry (DSC) [EM3, EM4]; and above the phase transition and in their dry state by thermal analysis [EM2], solid state NMR spectroscopy [EM3, EM4], X-ray powder diffraction (XRD) [EM1] and high resolution transmission electron microscopy (HRTEM).
Results
Investigating the swelling degree is a simple method that can indicate drug - polymer interactions. The applied model molecules have extremely different effect on the swelling behaviour of hydrogel. OH-substituted phenols reduce the swelling degree at any case. Phase transition takes place yet under 34 °C, at a “critical” concentration (ccrit) that is characteristic of the number and relative position of the OH groups (Fig.5). For meta phenols (phenol, 1,3-dihydroxybenzene and 1,3,5-trihydroxybenzene) more hydroxyl groups result in a lower, for ortho phenols (phenol, 1,2-dihydroxybenzene and 1,2,3-trihydroxybenzene), a higher ccrit. Phenols reduce the temperature of phase transition proportionally to their concentration.
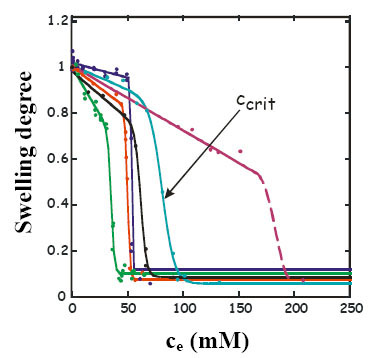
Fig.5 Swelling degree of PNIPA gel in different phenolic solutions at 20 °C as a function of equilibrium concentration in the free liquid phase: ●1,3,5-trihydroxybenzene ●1,3-dihydroxybenzene ●phenol ●1,4-dihydroxybenzene ●1,2-dihydroxybenzene ●1,2,3-trihydroxybenzene [EM1]
Contrary to phenols, no transition occurs even in very concentrated (500 mM) solutions of dopamine and ibuprofen. Dopamine has no detectable influence on swelling; and ibuprofen causes only a minor decrease [EM1,EM4]. Dopamine slightly increases the temperature of phase transition, while ibuprofen has virtually no effect on it [EM1,EM4]. 1H-1H CRAMPS (Combined Rotation and Multiple Pulse Spectroscopy) NMR spectra revealed strong hydrogen bonding between phenol molecules and the side chains of the polymer. Interactions between dopamine and the PNIPA molecules could not be detected, but it was found that dopamine molecules interact preferentially with other dopamine molecules [EM3]. In case of ibuprofen only a part of the drug molecules interact with PNIPA, others stay in a free state. Consequences of these interactions could be clearly identified by thermal analysis [EM2].
Drug - polymer interactions significantly affected the drying process of the loaded gels, and so the crystalline state and crystallite size of drug molecules in the dry gel matrices. According to XRD spectra, the hydrogen bridges between phenol and PNIPA [EM4] hinder the crystallization during the drying process, giving rise to amorphous phenol [EM1]. In the dopamine loaded gel gradually developing crystals were observed, illustrating how the lack of drug - polymer interactions and the strong dopamine - dopamine interaction foster crystallization [EM1]. Although hydrogen bonding between ibuprofen and PNIPA does occur [5], according to my NMR results only some of the ibuprofen molecules interact with PNIPA, thus allowing the fast crystallization of non-interacting drug molecules. (Fig.6). The size of dopamine and ibuprofen crystallites inside the gel is some ten nanometres in contrast with those developed in free state and are above 100 nm. As loading into a gel matrix significantly influences the crystallinity and particle size of the stored drugs, the investigated dry drug carrier systems may be suitable not only for retard release, but also for enhancing the dissolution and bioavailability of drugs.
The drug content changes the swelling degree of loaded gels. The swelling degree of the loaded gel is higher for phenol, unaltered for dopamine and lower for ibuprofen compared to the pure gel. This explains that 100 % of the phenol, 91 % of the dopamine and 75 % of the ibuprofen molecules were released from the gel in a 10 hour experiment. During the reswelling process, the changes in the interactions influence the rate and kinetics of release.
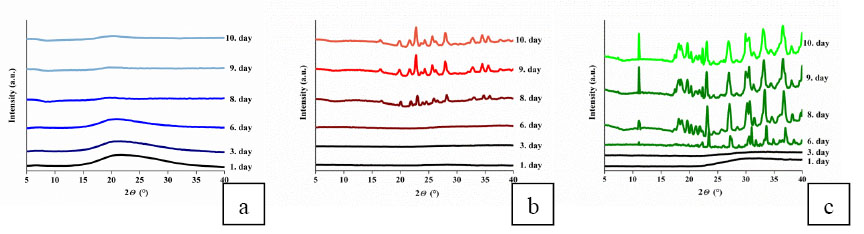
Fig.6 XRD spectra of the guest - PNIPA systems during the drying process: (a) phenol loaded PNIPA (b) dopamine loaded PNIPA (c) ibuprofen loaded PNIPA. Successive curves are shifted vertically for clarity. [EM1]
Expected impact and further research
My results may contribute to the development of retard drug formulae based on the shape memory of responsive gels. Currently we are extending our drug delivery investigations to hybrid gels containing carbon nanoparticles. By incorporating carbon nanotubes (Fig.7) and graphene(oxide) into the PNIPA polymer, advanced transport systems can be prepared with enhanced mechanical strength and drug uptake, tuned swelling and release properties. The absorption of carbon nanoparticles in the infra range enables the control of swelling by infrared light.

Fig.7 Hybrid PNIPA gels with various carbon nanotube content
Publications, references, links
Related publications
(IF = impact factor)
[EM1] E. Manek, A. Domján, J. Madarász, K. László: Interactions in aromatic probe molecule loaded poly(N-isopropylacrylamide) hydrogels and implications for drug delivery. European Polymer Journal 2015;68:657-664 (IF 3.005)
[EM2] E. Manek, A. Domján, A. Menyhárd, K. László: Host-guest interactions in poly(N-isopropylacrylamide) gel: a thermo-analytical approach. Journal of Thermal Analysis and Calorimetry 2015;120(2):1273-1281 (IF 2.042)
[EM3] A. Domjan, E. Manek, E. Geissler, K. László: Host-Guest Interactions in Poly(N-isopropylacrylamide) Hydrogel Seen by One- and Two-Dimensional 1H CRAMPS Solid-State NMR Spectroscopy. Macromolecules 2013;46(8):3118-3124 (IF 5.800)
[EM4] K. László, E. Manek, Sz. Vavra, E. Geissler, A. Domján: Host-guest Interactions in Poly(N-isopropylacrylamide) Hydrogels. Chemistry Letters 2012;41(10):1055-1056 (IF 1.300)
[EM5] E. Manek, A. Menyhárd, A. Domján, K. László. Szimultán termikus analízis a hatóanyagleadás kutatásának szolgálatában. MTA Termoanalitikai Munkabizottságának Ülése (in Hungarian), 2014.12.10, Budapest
[EM6] E. Manek, A. Domján, A. Menyhárd, J. Madarász, K. László. Host-guest interactions in poly(N-isopropylacrylamide) gel seen by thermal simultaneous thermal analysis and powder X-ray diffraction method. 12th International Symposium on Bioscience and Nanotechnology, 2014.11.14-15, Toyo University, Kawagoe
[EM7] E. Manek, A. Domján, A. Menyhárd, K. László. Termikus módszerek a hatóanyagleadás-kutatás szolgálatában. MKE Kozmetikai és Háztartásvegyipari Társasága, Kozmetikai Szimpózium (in Hungarian), 2014, 2014.11.13, Budapest. Lectures Summaries, p.12, ISBN 978-963-9970-51-9
[EM8] E. Manek, A. Domján, J. Madarász, K. László. Hidrogélek és hatóanyagmolekulák kölcsönhatásának szerepe a tervezett hatóanyagleadásban. MKE Kozmetikai és Háztartásvegyipari Társasága, Kozmetikai Szimpózium (in Hungarian), 2014, 2014.11.13, Budapest. Lectures Summaries, p.13, ISBN 978-963-9970-51-9
[EM9] E. Manek, A. Domján, J. Madarász, T. Sovány, K. László. Interactions of poly(N-isopropylacrylamide) hydrogels with small aromatic molecules of environmentally and biomedically relevance. 2nd International Conference on Bio-based Polymers and Composites (BiPoCo 2014), 2014.08.24-28, Visegrád, Hungary
[EM10] K. László, E. Manek, Sz. Vavra, E. Geissler, A. Domján. Host-guest interactions in poly(N-isopropylacrylamide) hydrogels. Conference of the International Association of Colloid and Interface Scientists 2012 (IACIS 2012), 2012.05.13-18, Sendai
Links
References
[1] K. Kosik, E. Wilk, E. Geissler, K. László. 2007;40(6):2141-2147
[2] K. László, K. Kosik, E. Wilk, E. Geissler. NATO ASI series. Springer 2006;393-402
[3] A. Domján, E. Geissler, K. László. Soft Matter 2010;6:247-249
[4] C. Hofmann, M. Schönhoff. Colloid Polymer Science 2009;287:1369-1376
[5] H. Jiang, L. Wang. Chinese Chemical Letters 2011;22:1123–1126
