|
|
BMe Research Grant |
|
Doctoral School of Psychology (Cognitive Science)
Department of Cognitive Sciences
Supervisor: Prof. Kovács Gyula
The complex behavioral and neural investigation of developmental prosopagnosia
Introducing the research area
The research of face blindness (prosopagnosia) has developed dynamically in the past 10-15 years. Recent studies (Kennerknecht et al., 2006, 2008) showed that the developmental prosopagnosia seems to affect 2-2,5% of the population irrespective of gender and race. In the most severe cases of face blindness, even identifying the close relatives is impossible. Due to it’s high prevalence, the exact understanding of psychological and neural basis of prosopagnosia is not merely a scientific question. This might form the basis of work toward new therapeutic approaches.
History and context of the research
The research of face recognition in the electrophysiology laboratory of the Department of Cognitive Science at the BUTE focuses on the behavioral and neural background of face processing. In our research, we apply behavioral, electrophysiological (EEG), neuroimaging (fMRI) techniques and eye-tracking system in neurotypical population. In addition to the investigation of normal face-related processes, in 2010 we started studying the neural aspects of face blindness. Devices were used in the research correspond to modern trends and were purchased with the support of the current topic related tender of the OTKA (Hungarian Scientific Research Fund).
History and context of the research
As frequently mentioned, faces are special stimuli. In addition to the social aspects of faces there are underlying psychological and neuroscientific pieces of evidence. One of them comes from neuropsychology. There are individuals with difficulties in face recognition without problems with non-face objects recognition and conversely, there are individuals with intact face recognition and problems with object recognition, thus these mechanisms are functionally dissociative. Face blindness became well known in the scientific community after 1947 when a German neurologist, Bodamer reported cases of three soldiers suffered brain damage. These soldiers were unable to recognize their relatives and friends, however, one of them was able to recognize some faces by the characteristic hair and moustache. Besides the acquired type of prosopagnosia (AP) caused by localized brain damage due to the injury or infection of the central nervous system, the developmental form of the disease (developmental prosopagnosia – DP) first described by McConachie in 1976 – can be inherited, and occurs without any external impact. To date, despite the increasing number of studies using different techniques, the unitary classification of prosopagnosia is not resolved due to the heterogeneity of the disease. In 2010, we have initiated the systematic investigation of the neural background of DP in three members of a family presenting with face blindness (father, son, daughter). Although investigation of patients from a common ancestor would be extremely helpful to discover the genetic background of the prosopagnosia, analysis of family members with prosopagnosia is quite a rarity in the literature. In 2011, we have initiated a project to study the developmental prosopagnosia recruiting a group consisting of prominently high number of participants presenting with DP.
The research goal, open questions
It is well known that different types of the neural diseases may lead to similar problems in face recognition, however, there are situations where similar neural problems may cause heterogeneous symptoms. The heterogeneity of the disease is emphasized frequently in the majority of recent studies. The main aim of our studies was to evaluate the neural mechanisms that play crucial role in the variety of the behavioral outcome. Furthermore, we thought that revealed mechanisms will help correctly categorize the cases into different subtypes of DP. In spite of Behrmann et al. (2010) we think that different types of the disease in the central nervous system may lead the same diagnose: prosopagnosia.
Both theoretically and based on the literature of DP, it seems impossible to identify a single feature responsible for the evolution of the disease at neural level. Still there is a huge demand for a common theoretical framework that enables the integration of the divergent features of the disease. To validate such a system, a 30-assay assessment and matched controls with accordant criteria would be adequate. To our best knowledge, this type of investigation has not been completed as yet.
Using an extraordinary sample size, our aim is to create a valid spectral system for face recognition, where we can characterize the various phenotypes together with the severity of disease at individual level. Furthermore, we aimed to study the genetic background of prosopagnosia.
Methods
To explore the nature of face blindness, we apply several behavioural tests. The outcome of these trials formed the basis of further questions and analysis of the disorder with the conventional apparatuses of cognitive neuroscience. To resolve the controversial findings in literature and for a systematic analysis of the disease, a huge sample size is of particular necessity. Such an extraordinary sample size is unique in the research field of the face blindness, thus our first and most important ambition was to generate one. In 2011, we set up a public website that provides information about the prosopagnosia and the Hungarian adaptation of the questionnaire earlier used in the 2 largest sample assays, were also made available here. To promote our efforts, an article including a description of our research group and the accessibility of the questionnaires was published on the national Internet portal on June 4th, 2012. Until now, more than 2000 people answered the questionnaire. As a result of the wide interest several media outlets sought our group for interviews and further information about the project.
In accordance with the results of Kennerknecht et al. (2006, 2008) we assumed that the disorder affects 2-2.5% of the population.
After the exclusion of lower-level abnormalities of object recognition with appropriate test batteries (Ishihara Color blindness test (Ishihara, 1925); Doors & People (Baddeley et al., 1994); Birmingham Object Recognition Battery: BORB, Riddoch & Humphreys, 1993) we investigated the face-specific processes of the volunteers in the Electrophysiological Laboratory of the Dept. of Cognitive Science at the BUTE. The experimental procedure took 8 hours for every subjects. This test battery consists of internationally validated behavioural assays: (Cambridge Famous Faces Test: CFFT (Duchaine & Nakayama, 2005), Cambridge Face Memory Test: CFMT (Duchaine and Nakayama, 2006), Cambridge Face Perception Test: CFPT (Duchaine et al., 2007), Cambridge Car Memory Test: CCMT (Dennett et al., 2012), Philadelphia Face Perception Battery: PFPB (Thomas et al., 2008), Faceparts-matching test (self-designed and validated)) and an EEG session comprising of different experiments.
Our final goal with all these assessments was to create a universal face-recognition profile that involves previous models of prosopagnosia and is suitable for the detailed discrimination of inter-individual differences, both in the prosopagnosic and the neurotypical population. To date we have fully completed all test batteries on 35 subjects presenting with developmental prosopagnosia and also with individually matched controls.
EEG and evoked potential (EP) experiments were performed in the electrophysiological laboratory of the BUTE using a 64 channel EEG system (BrainProducts GmbH, Munich, Germany). The fMRI measurements were performed in the MR Research Center (Szentágothai Knowledge Center, Semmelweis University) using 3 Tesla-s Achieva machine (Philips, Best, Netherlands). The fMRI analysis was performed using SPM 8.0 (SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK)), the electrophysiologic data were analyzed using Vision Analyzer 1.1 (BrainProducts GmbH, Munich, Germany) and custom-written Matlab 7.6 (Mathworks, Natick Massachusetts, USA) software.
Current results
By all the three members of the prosopagnosic family we have found serious face-selective behavioral impairment. Deficiency of perceptual processes may account for this phenomenon. Sub-control achievements in face recognition tests support this assumption.
The results of EP analysis were in line with the behavioural abnormalities we found. The amplitude of face selective evoked potential N170 (a negative EEG slope appearing 170 milliseconds after exposing to visual stimuli, Bentin et al., 1996; Rossion & Jacques, 2008) showed no face-selective differences evoked either by face or noise stimulus in the members of the family (Németh et al., 2014d).
This phenomenon is present only in few subjects in the new sample.
Spectrum analysis and sign-coherence assessments of the EEG data of the family members revealed that the absence of face-recognition does not result from the decrease of face-evoked responses. It originates from the increase of the amplitude of noise-responses and due to the increased synchronicity of N170 latency (Fig. 1; Németh et al., 2014d).
By these individuals, noise stimulation is followed by a more synchronous 4-12 Hz cortical activity that indicates the lack of the selectivity of face-processing neuronal cell population in developmental prosopagnosia.
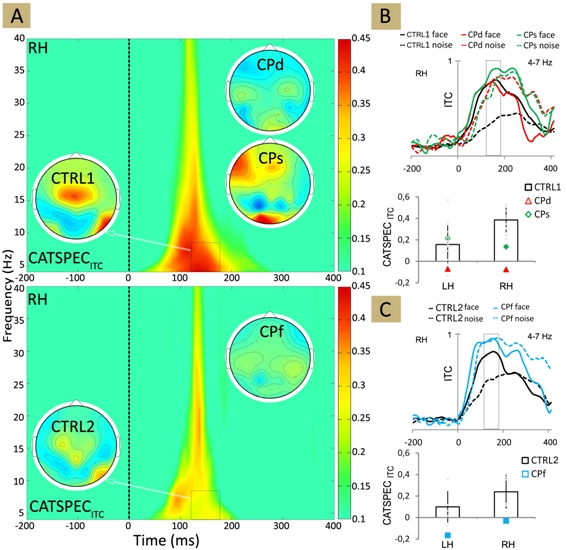
Figure 1. (A) The plot showing the difference in ITC (intertrial coherence) for face minus non-face stimuli for CTRL1 (upper panel) and for CTRL2 (lower panel). Insets show the ITC topographies averaged over 130-190 ms and 4-7 Hz as indicated by the black dashed boxes. (B)-(C) Top: Mean theta-band ITC for face and noise stimuli. Bottom: The average difference in theta-band ITC for face minus non-face stimuli, calculated in the 130-190 ms time window for CTRL1 (B) and for CTRL2 (C). Black: CTRL1 and CTRL2; red: CPd, green: CPs, blue: CPf. Larger and filled symbols in the diagrams mark significant differences from the values of the matched neurotypicals. Error bars denote the 95% bootstrap confidence interval. LH - left hemisphere, RH - right hemisphere.
Imaging study on the family members confirmed the injury of CORE brain areas responsible for sensory face processing (Haxby et al., 2002). This supports the establishment of perceptual-based prosopagnosia diagnosis (Fig. 2.; Németh et al., 2014b).
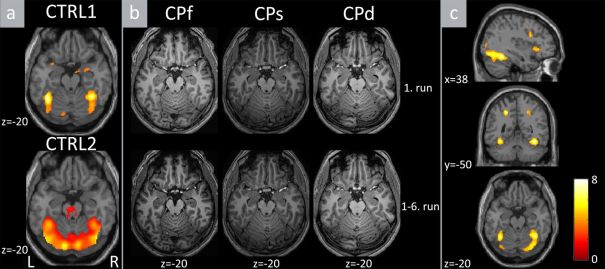
Figure 2. The results of the whole-brain analyses. (a) The results of the face vs. object + noise contrast for the young CTRL1 (upper panel) and for the older CTRL2 (lower panel) groups. (b). The results of the same contrast separately for CPf, CPs and CPd. Upper panels depict the activations of the 1st run. Lower panels depict the same contrast in all the recorded 6 runs to increase the signal-to-noise ratio. (c). Group-wise random level differences of the same contrast between controls (CTRL1 and CTRL2 merged) and CP subjects (CPf, CPs, CPd). L - left, R - right.
The 35 recently recruited individuals showed significantly decreased performance in face perception and face recognition behavioral tests, with varied severity. This also support our spectral hypothesis.
Current results collected from the prosopagnosic family and from the new assay, suggest the existence of possible subtypes of developmental prosopagnosia. Different DP types can be characterized by the evaluation of the effectivity of noise processing system. Therefore, as a part of the EP investigations we revealed the EEG correlates of noise processing.
We did not identified category-sensitive ERPs (N170, P2) in connection with the noise processing mechanisms in the neurotypical brain (Németh et al., 2014a). The publication of the most current results are in progress (see Németh et al., 2014c; Zimmer et al., 2014).
Behavioral data and the variable category-sensitive N170 ERP component of prosopagnosic individuals suggest the existence of different types of the disease (Németh et al., 2014c).
According to our recent results, for a considerable part of the new prosopagnosic group (22 subjects) the disease is presumably connected with the late, memory-related part of the stimulus processing mechanisms. In other words, this type of the disease may be considered as associative prosopagnosia (DeRenzi et al., 1991). In the remaining part of the prosopagnosic group (13 subjects), the problems may be caused by the errors at the perceptual stage of stimulus processing (Fig. 3.).
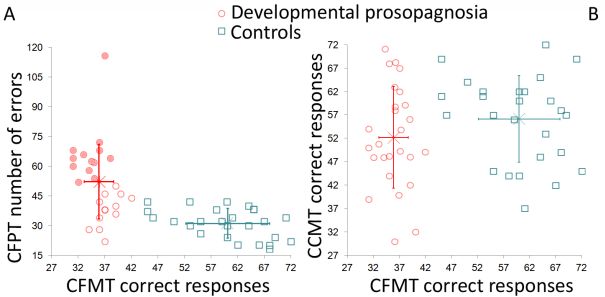
Figure 3. The diagrams show the results of different visual tests of subjects (only in the range of 20-40 years) presenting with developmental prosopagnosia (red circles) and matched controls (blue squares). (A) The results of the Cambridge Face Memory Test (CFMT; diagnostic test of prosopagnosia) and the Cambridge Face Perception Test (CFPT). The values of the CFMT discriminate individuals with prosopagnosia and neurotypicals, while CFPT error scores suggest two subtypes of prosopagnosia (more errors - apperceptive, filled circles; less errors - associative; empty circles). (B) The scores of the CFMT and the number of correct responses on the Cambridge Car Memory Test (CCMT). Equal scores on the CCMT in prosopagnosia and control group suggest that face recognition difficulties of individuals with prosopagnosia can not be accounted for general perceptual or non-face selective problems. Error bars denote group means and the 95% confidence intervals.
Expected impact and further research
Our recent results support our spectral prosopagnosia hypothesis, namely individuals suffering from inability of face perception and recognition are localized at low levels of a wide face-recognition spectrum characteristic for the neurotypical population.
Our plan is to analyse and publish the data on the new cases of DP. These results may help develop individualized therapies and compensatory strategies against prosopagnosia.
In the last 3 years, we have published our results in both international and Hungarian journals and presented our posters and demonstrations at conferences. Taken together, the amount, quality and relevance of our results implicate further publications in the coming years.
Publications, references, links
Posters and presentations
- Németh, K., Zimmer, M., Albu, M., Nagy, K., Kovács, Gy. (2011). Ismeretlen ismerősök - az örökletes arcfelismerési zavar kísérleti vizsgálata (in Hungarian) (XV. Pszinapszis - "Határok nélkül": Jövő kutatói szekció - Budapest (April 15-17, 2011) - presentation
- Németh, K., Zimmer, M., Albu, M., Nagy, K., Kovács, Gy. (2011). Az öröklődő prosopagnosia az arcfeldolgozás korai lépéseinek zavara (in Hungarian) (A Magyar Pszichológiai Társaság XX. Országos Tudományos Nagygyűlése - Budapest (May 25-27, 2011) - presentation
- Németh, K. (2011). Prosopagnosia - arcvakság (in Hungarian) (I. BME Doktorandusz Konferencia - Budapest (Nov. 25, 2011)) - presentation
- Németh, K., Zimmer, M., Albu, M., Nagy, K., Bankó, É., Vidnyánszky, Z., Kovács, Gy. (2012). The response dynamics of occipito-temporal areas is altered in congenital prosopagnosics (IBRO International Workshop 2012 - Szeged, Hungary (Jan. 19-21, 2012)) - poster
- Németh, K., Zimmer, M., Bankó, É., Vidnyánszky, Z., Kovács, Gy. (2012). The response dynamics of occipito-temporal areas is altered in congenital prosopagnosics (ECVP 2012 - European Conference on Visual Perception - Alghero, Italy (Sept. 2-6, 2012)) - poster
- Zimmer, M., Németh, K., Kovács, Gy. (2012) Electrophysiological study of congenital prosopagnosia (ECVP 2012 - European Conference on Visual Perception - Alghero, Italy (Sept. 2-6, 2012)) - poster
- Kovács, P., Németh, K., Kovács, Gy., Vakli, P., Zimmer, M. (2013). Noise-induced modulation of the event-related potential is similar for various high-level stimulus categories. (V. Dubrovnik Conference on Cognitive Science – DuCog 2013) - poster
- Németh, K., Kovács, P., Vakli, P., Zimmer, M., Kovács, Gy. (2014). SSVEP identity adaptation relates to inter-individual differences of face perception (PPRU - Workshop IX. - From cortical areas to social functions - Jena, Germany (Apr. 4-6, 2014) - poster
- Kovács, P., Németh, K., Kovács, Gy., Vakli, P., Zimmer, M. (2013). A vizuális zaj a kiváltott válaszok korai kategóriaspecifikus modulációját mutatja (in Hungarian) (18. Magyar Látás Szimpózium - Pécs, Hungary (Dec. 19, 2013)) - presentation
- Németh, K. (2013). Pont, pont, vesszőcske...(in Hungarian) Vírus Pszicho Est (Feb. 13, 2013) - presentation
- Németh, K. (2013). Arcvakság. Vírus Best of (Jul. 27, 2013) - presentation
- Németh, K., Kovács, P., Vakli, P., Kovács, Gy., Zimmer, M. (2014c). Subtypes of developmental prosopagnosia: the role of the perceptual and memory factors (ECVP 2014 - Belgrad, Serbia (Aug. 24-28, 2014) - poster
- Zimmer, M., Németh K., Kovács, P., Vakli P., Kovács, Gy. (2014). Noise-induced perceptual processing demands in developmental prosopagnosia (ECVP 2014 - Belgrad, Serbia (Aug. 24-28, 2014) - poster
Publications
- Németh, K., Zimmer, M., Schweinberger, S. R., Vakli, P., Kovács, Gy. (2014d). The background of reduced face specificity of N170 in congenital prosopagnosia. Plos ONE. (IF: 3.73) doi: 10.1371/journal.pone.0101393
- Németh, K., Kovács, P., Vakli, P., Kovács, Gy., Zimmer, M. (2014a). Noise reveals early category specific modulation of the event-related potentials. Frontiers in Psychology, 5:367. doi: 10.3389/fpsyg.2014.00367
- Németh, K., Zimmer, M., Nagy, K., Bankó, É., Vidnyánszky, Z., Vakli, P., Kovács, Gy. (2014b). Altered BOLD response within the core face processing network in congenital prosopagnosia. Clinical Neuroscience. (IF: 0.35)
- Vakli, P., Németh, K., Zimmer, M., Schweinberger, S. R., Kovács, Gy. (2014). Altering second-order configurations reduces the adaptation effects on early face-sensitive event-related potential components. Frontiers in Human Neuroscience, 8:426. (IF: 2.9) doi: 10.3389/fnhum.2014.00426
- Németh, K., Zimmer, M. (in prep.). Az arcfelismerési zavarok osztályozása a kialakulás oka, az idegtudományi, valamint a viselkedéses eredmények tükrében. Magyar Pszichológiai Szemle
- Vakli, P., Németh, K., Zimmer, M., Kovács, Gy. (in prep.). The face evoked steady-state visual potentials are sensitive to the orientation, viewpoint, expression and configuration of the stimuli. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2014.10.008 (IF: 2.648)
- Kovács, P., Németh, K., Kovács, Gy., Vakli, P., Zimmer, M. (2013). Noise-induced modulation of the event-related potential is similar for various high-level stimulus categories. Learning & Perception. Volume 5. pp. 47.
- Vakli, P., Németh, K., Zimmer, M., Schweinberger, S. R., Kovács, Gy. (2012). Face distortion aftereffects evoked by featureless first-order stimulus configurations. Frontiers in Psychology, 3:566.
- Németh, K., Zimmer, M., Bankó, É., Vidnyánszky, Z., Kovács, Gy. (2012). The response dynamics of occipito-temporal areas is altered in congenital prosopagnosics. Perception, Vol. 41. Supplement, pp. 115.
- Zimmer, M., Németh, K., Kovács, Gy. (2012). Electrophysiological study of congenital prosopagnosia. Perception, Vol. 41. Supplement, pp. 250.
- Vakli, P., Németh, K., Zimmer, M., Kovács, Gy. (2012). Steady-state visual-evoked potential adaptation to faces is invariant to orientation, viewpoint and emotions. Perception, Vol. 41. Supplement, pp. 184.
Links
- Arcvakság – egy ritka(?) neuropszichológiai zavar
- Felismersz? - a normál arcfelismerés folyamatai
- BUTE Dept. of Cognitive Science
- BUTE Dept. of Cognitive Science - the website of prosopagnosia study
- Prosopagnosia questionnaire
- Prosopagnosia short questionnaire
- Ötvenből egy ember nem ismer fel arcokat
- Közelről: arcvakság
References
- Baddeley AD, Emslie H, Nimmo-Smith I (2004) Doors and People: A Test of Visual and Verbal Recall and Recognition. Bury St. Edmunds, UK: Thames Valley Test Company.
- Behrmann, M., Avidan, G., Thomas, C., & Humphreys, K. (2010). Congenital and Acquired Prosopagnosia: Flip sides of the same coin? In I. D. Bub, I. Gauthier & M. Tarr (Ed.), Perceptual Expertise: Bridging Brain and Behavior (pp. 167-196): Oxford University Press.
- Bentin S, Allison T, Puce A, Perez E, McCarthy G (1996) Electrophysiological Studies of Face Perception in Humans. Journal of Cognitive Neuroscience 8: 551-565.
- Bodamer, J. (1947). Die Prosop-Agnosie. Archiv für Psychiatrie und Nervenkrankheiten, 179, 6–53.
- Dennett, H. W., McKone, E., Tavashmi, R., Hall, A., Pidcock, M., Edwards, M., & Duchaine, B. (2012). The Cambridge Car Memory Test: a task matched in format to the Cambridge Face Memory Test, with norms, reliability, sex differences, dissociations from face memory, and expertise effects. Behav Res Methods, 44(2), 587-605.
- De Renzi E, Faglioni P, Grossi D, Nichelli P. Apperceptive and associative forms of prosopagnosia. Cortex 1991; 27: 213–22.
- Duchaine, B., & Nakayama, K. (2005). Dissociations of face and object recognition in developmental prosopagnosia. Journal of Cognitive Neuroscience, 17, 249–261.
- Duchaine, B., & Nakayama, K. (2006). The Cambridge Face Memory Test: results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia, 44(4), 576-585.
- Duchaine B, Germine L, Nakayama K (2007) Family resemblance: ten family members with prosopagnosia and within-class object agnosia. Cognitive Neuropsychology 24: 419-430.
- Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 51(1), 59-67.
- Ishihara, s. (1925). Tests for Colour-Blindness. Tokyo: Kanehara.
- Kennerknecht, I., Grueter, T., Welling, B., Wentzek, S., Horst, J., Edwards, S., & Grueter, M. (2006). First report of prevalence of non-syndromic hereditary prosopagnosia (HPA). American Journal of Medical Genetics Part A, 140(15), 1617-1622.
- Kennerknecht, I., Ho, N. Y., & Wong, V. C. (2008). Prevalence of hereditary prosopagnosia (HPA) in Hong Kong Chinese population. American Journal of Medical Genetics Part A, 146A(22), 2863-2870.
- McConachie HR (1976) Developmental prosopagnosia. A single case report. Cortex 12: 76-82.
- Németh, K., Kovács, P., Vakli, P., Kovács, Gy., Zimmer, M. (2014a). Noise reveals early category specific modulation of the event-related potentials. Frontiers in Psychology, 5:367. doi: 10.3389/fpsyg.2014.00367
- Németh, K., Zimmer, M., Nagy, K., Bankó, É., Vidnyánszky, Z., Vakli, P., Kovács, Gy. (2014b). Altered BOLD response within the core face processing network in congenital prosopagnosia. Clinical Neuroscience.
- Németh, K., Kovács, P., Vakli, P., Kovács, Gy., Zimmer, M. (2014c). Subtypes of developmental prosopagnosia: the role of the perceptual and memory factors (ECVP 2014 - Belgrad, Serbia (Aug. 24-28, 2014) - poster
- Németh, K., Zimmer, M., Schweinberger, S. R., Vakli, P., Kovács, Gy. (2014d). The background of reduced face specificity of N170 in congenital prosopagnosia. Plos ONE. doi: 10.1371/journal.pone.0101393
- Rossion B, Jacques C (2008) Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage 39: 1959-1979.
- Thomas, A. L., Lawler, K., Olson, I. R., & Aguirre, G. K. (2008). The Philadelphia Face Perception Battery. Archives of Clinical Neuropsychology, 23(2), 175-187.
- Riddoch, M. J., & Humphreys, G. W. (1993). Birmingham object recognition battery. LEA, Hove, UK.
- Zimmer, M., Németh K., Kovács, P., Vakli P., Kovács, Gy. (2014). Noise-induced perceptual processing demands in developmental prosopagnosia (ECVP 2014 - Belgrad, Serbia (Aug. 24-28, 2014) - poster
