 |
BMe Research Grant |

|
Doctoral School of Electrical Engineering
Department of Electronics Technology
Supervisor: Prof. Gábor Harsányi
Investigation on electrochemical migration in microelectronics
Introducing the research area
My research field is focused on the electrochemical migration (ECM) phenomenon, with the general aim to improve the reliability of electronics. The common characteristics of the ECM phenomenon include the presence of moisture on conductor-dielectric-conductor systems under bias voltage, the electrochemical process and the metallic dendrite growth. This process is driven by an applied electric field from the anode to the cathode. Dendrite growth occurs as a result of metal ions being dissolved into a solution. These escape from the anode and are deposited at the cathode, growing in needle- or tree-like formations (Fig. 1). This effect causes short-circuits in the electronic circuits, which may lead to a catastrophic failure.
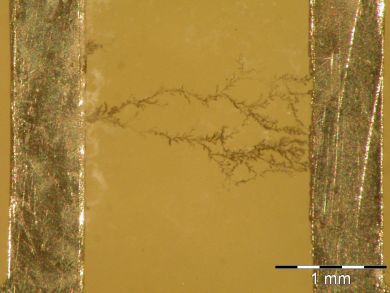
Figure 1. Short circuit caused by a Sn dendrite.
Brief introduction of the research place
The main focus of the research center – according to the Department's mission – is on the investigation of the material and technological aspects of physical connections between microcircuits and larger systems by involving the knowledge on manufacturing, quality and reliability. Due to the growing demands in the electronics industry for miniaturization and increased integration and thus the growing density of conductive layers and the decreasing distances between the leads of electronic components, smaller and smaller areas have to be investigated by using interdisciplinary knowledge. For this reason, our research center is equipped with special equipments which can investigate solid phase conductive, insulating and semiconductor structures and, in some cases, can even carry out liquid-phase measurements between all scales from the human visibility to nano sizes. For these purposes, the department has optical microscopes (Olympus SZX9 and BX51), an X-ray microscope (Dage XiDAT 6600), a scanning electron microscope (FEI Inspect S50) with an energy disperse X-ray spectrometer unit (Bruker Quanta EDX), a scanning acoustic microscope (Sonix HS 1000), X-ray fluorescence spectroscopy (Spectro Midex M), and an atomic force microscopy (Veeco diInnova). In order to perform investigations under harsh environments, different environmental chambers can be applied; a Highly Accelerated Stress Test chamber (Tabai Espec); two Thermal-Humidity Chambers (EHS-211M and Weiss, WK 180/40) (see Fig. 2.), and a Thermal Shock Chamber (Tabai Espec, TSE-11-A).

Figure 2. Thermal-Humidity Chamber
History and context of the research
Nowadays, because of the keen competition in the electronic industry, customer requirements must be ranked first. Therefore, it is increasingly important to ensure the reliability and quality of electrical devices by using cost effective technologies. Depending on the applications, the requirements of customers are different, but all of them would like to know whether the lifetime, quality and reliability of the devices will meet their requirements.
From the aspect of ECM reliability problems, the main topics are investigations of physical and chemical behaviors on different surface finishes, solder alloys and joints. The Restriction of Hazardous Substances (RoHS) operative directive [1] has just increased the complexity of this challenge as lead bearing solders have to be replaced with lead-free ones. In this context, alternative binary alloys have been examined as replacements for SnPb (traditional) solders, such as near-eutectic SnAg, SnCu, and SnZn alloys. However, ternaries (SnAgCu, SnZnAg, SnZnIn, etc.) and even quaternary alloys (SnZnAgAl, SnAgBiCu, SnInAgSb) have also been studied as candidates for lead-free solders. The reliability investigation of lead-free solders is still a very current issue among the researchers, and one of the important topics is electrochemical migration (ECM) failure phenomenon [2, 3].
The first theoretical explanation of the phenomenon was given in connection with the silver migration [4]. In this migration model, dendrites are grown on the cathode by the deposition of anodically dissolved ionic constituents that are present in the conductive strips. The migrated resistive shorts occur at practically random locations, and emerge mainly under extreme conditions (i.e. high temperature and humidity). However, a number of electrical field-governed failure types are correlated with migration. A device can operate for many hundreds of hours under normal operating conditions, and then, after a short exposure to special environmental conditions, it may fail suddenly [4].
Later it was proven in numerous further studies that several other metals also show dendrite growth caused by ECM, i.e. Cu, Pb and Sn. Furthermore, it was also proven that ionic contaminants have a high impact on ECM as well. Nevertheless, the presence of new lead-free based solder materials generated an increasing need to investigate them for the ECM phenomenon. Even recent publications have pointed out the importance of this research field [5, 6].
The research goal, open questions
According to the state of the art, the ECM phenomenon has got still many open questions such as undefined reactions and processes. The existing models cannot adequately describe the whole process of ECM. The interpretation will be difficult indeed, due to the novel lead-free solder alloy systems which involve different electrochemical processes.
These electrochemical processes occur in very small amounts of electrolytes (~µl), furthermore the processes are not stationary and the systems are not homogeneous as well. Therefore, there are no descriptions of equations, only empirical models exist [7].
Usually, the investigations are carried out in two different scenarios: one of them is done under laboratory circumstances (at room temperature and humidity), while the other one is carried out in extreme climatic conditions.
The latter one is carried out by environmental tests, which provide high temperature and humidity levels, while the patterns are followed by some kinds of electrical parameters, such as surface insulation resistance, leakage current or voltage changing.
However, the environmental tests provided data mainly about the ECM processes (fault detection) and gave very little useful information about the antecedent processes such as water condensation mechanism.
Although environmental chambers (tests) can simulate the mechanism of water condensation, there is little information available about the physical aspects of condensation which leads to ECM and dendrite growth [8].
Another open question is the impact of condensation time on the mean time failure time (MTTF); it can be supposed that the time ratio between condensation time and electrochemical migration time (dendrite growth) is not irrelevant in certain cases.
1. The first aim of my research was to gain a better understanding of the antecedent processes of ECM short formation. Therefore, the effect of condensation on the total failure mechanism had to be clarified. Thus an improvement of a novel measuring method was aimed, which provides new information about ECM behavior, for example the water condensation process during Thermal-Humidity Bias (THB) tests.
2. The second aim addresses the ECM-related condensation mechanism. Supposedly, the condensation time (MTTC) strongly depends on the capillary effect and on other physical and chemical properties, such as wettability and/or water adsorption. In other words, the speed of MTTC could be mainly determined by the type of insulation material, since it can be supposed that in some cases the MTTC time has a close relevance to MTTF. If the hypothesis is proven, then some current migration models [4] will have to be modified with the MTTC.
3. Thirdly, there are still many contradictory statements in the literature. One of them is about the ECM susceptibility of Pb and Cu, where a rank of ECM susceptibility was established: Ag>Pb>Cu>Sn (Silver has the highest susceptibility for ECM) [9]. The aim of this investigation is to make easier the material choice for electrical circuit design, fabrication and reliability.
4. Finally, the last aim is focused on the ECM behavior of the novel lead-free solder materials. It is known that there is a huge difference in ECM behavior between the lead-bearing and lead-free solder alloys [10]. Therefore, comparative investigations of different solder alloys will be carried out focusing on ECM. The comparison can be based on the investigation of active and passive local cells on solder surface or structural and composition investigations of the intermetallic compound (IMC) formation.
Methodology
Environmental testing methods like THB test are the main investigating methods; however, the so called water drop (WD) test is also widely used at present. In case of the environmental tests, samples are stored in a climatic chamber where temperature is higher than room temperature and/or relative humidity is also high. This "aging" process is a common procedure in electronics for testing factors which, taken together or separately, influence the development of the reliability issues. However, it is less suitable for qualitative observation since the process is not as fast as the water drop test. Quantitative comparison is possible when the different samples are tested under identical conditions (relative humidity, temperature, voltage) and the changes of electrical parameters are monitored (e.g. insulation resistance or leakage current).
In WD test, a well-defined liquid drop (volume, concentration) is placed onto a conductive-dielectric-conductive structure, and a few volts (DC) is applied. Meanwhile, the formation of dendrites can be visually observed, the time dependence of the electrical parameter is measured and, finally, an average value is calculated (Mean-Time-To-Dendrite: MTTD). This is a very qualitative method as it does not model real circumstances. The advantage of this method is that the electrochemical migration susceptibility of metals can be easily and quickly established which makes it cost-effective as well.
Apart from environmental tests and WD tests, different surface analysis methods are also required. The surfaces of the samples are observed with scanning electron microscope (SEM). In this case, the aim is the topographical mapping of the surface (Fig. 3.) for which purpose magnifications up to 100,000x can be reached.

Figure 3. SEM image of a dendrite (green square= area of composition analysis)
The composition of the surface can be determined by element mapping by using the EDS (Energy-dispersive X-ray spectroscopy) method. EDS is a method available for determining the composition of materials at a defined point or area, which can present either atomic or mass ratio (Table 1).
|
Element |
[norm. mass %] |
[norm. atom %] |
Error (1 Sigma) |
|
Carbon |
15,1 |
55,2 |
2,5 |
|
Oxygen |
5,6 |
15,4 |
1,0 |
|
Tin |
79,3 |
29,4 |
2,2 |
Table 1. Composition of dendrites according to EDS method (green square)
X-ray Photoelectron Spectroscopy (XPS) and Scanning Auger Microscopy (SAM) are also applicable for ECM investigations. SAM is also known as Auger Electron Spectroscopy (AES). In this technique, the sample surface is bombarded with a high energy (3–10 kV) primary electron beam, which results in the emission of secondary, backscattered and Auger electrons from the area of bombardment and these can be readily detected and analyzed. The secondary and backscattered electrons are used for imaging purposes similar to scanning electron microscopy (SEM). The Auger electrons are emitted at discrete energies that are characteristic of the elements present on the sample surface. The characteristic energies of the Auger electrons are such that only the electrons from the outer 0.5 to 5 nm can escape and be detected. This makes SAM an extremely surface sensitive technique and the analysis volume is typically 106 to 108 times smaller than that excited in SEM/EDS analysis. All elements in the periodic table, except hydrogen and helium, can be detected and their concentration can be determined.
Results
My basic goal was to obtain more information about the electrochemical migration processes. Therefore, I have developed an innovative monitoring system, which allows "in-situ" and "real-time" observation of the process of water condensation and the subsequent shorting in extreme conditions (high temperature and humidity) on different surface conductor-insulator-conductor structures. The in-situ and real-time observation is possible within the following limits: -20 to +150 oC, at 100% relative humidity (see selected publications). In order to verify the measuring system, electric signal changes were measured and video recordings were made to gather information on water condensation and the formation of dendrites.
It was observed that the condensation was more intense on metals (Fig 4.). The initial small water droplets (primary nucleation) had swollen and suddenly formed from small water "islands" (secondary nucleation) into a continuous moisture film (Fig 5.), which sometimes spread all across the surface of the conductor-insulator-conductor structure (water bridges).
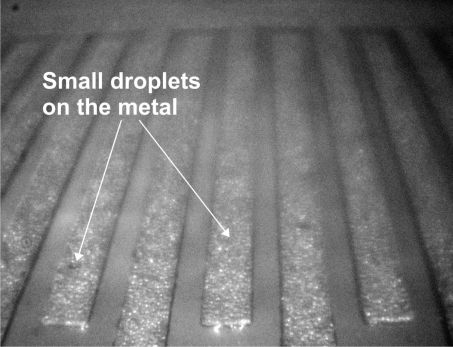
Figure 4. Start of condensation on the metal, while condensation cores one the insulation layer.
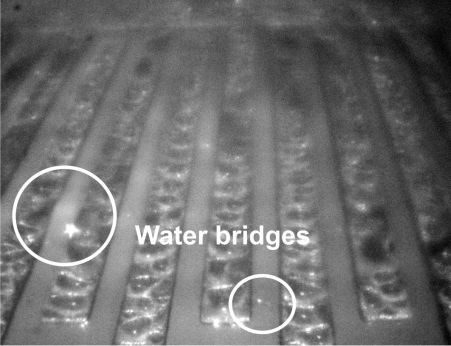
Figure 5. Appearance of water bridges due to even bigger droplets.
After this, the formation of dendrites (conductive filaments) could be observed, which then lead to shorts (Fig 6).
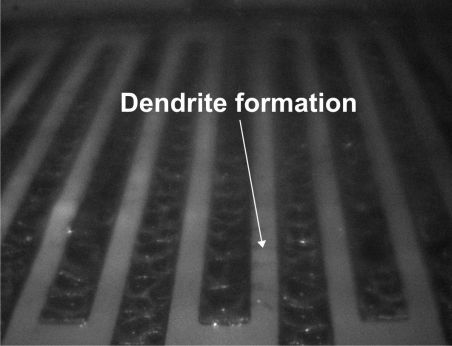
Figure 6. Dendrites formed after water bridges had occurred.
This observation is important, because it shows that water condensation processes taking place in the climatic chamber can have a strong influence on the short formation MTTF, depending on the materials used. However, the WD test ignores the MTTC time, which probably strongly varies with different conductor-insulator-conductor structures.
The so called capillary effect dominates the condensation on insulation layers (due to the surface free energy) [11], which phenomenon is the slowest process during condensation. Therefore, this impact mainly determines the MTTC, which results in water bridges (end of MTTC) and finally dendrite formations and shorts (MTTF).
The importance of the results is that the THB test gives a better model for the total ECM process than the WD test, since the latter eliminates the condensation mechanism. It can be supposed that different condensation times could be detected on various conductor-dielectric-conductor systems. According to the above mentioned results, the following can be stated:
I have introduced a novel in situ and real time monitoring system which is suitable for optical observations and simultaneous electrical parameter measurements during ECM processes inside climatic chambers. The monitoring system provides extended information about the ECM phenomenon, which is useful for a better understanding of the total ECM process (for more details, please see selected publications: a)).
It can also be supposed that the MTTC could significantly modify the Mean Time To Failure (MTTF), which includes the mean time to dendrite formation (MTTD) as well. In order to clarify the above mentioned hypothesis, two kinds of samples were fabricated and only one parameter was different between them: the type of the insulation layer, since – as I have mentioned above – the impact of the capillary effect has to be investigated with regard to MTTC and MTTF.
The two insulation materials were the widely used Kapton® polyimide (PI) and glass fiber-epoxy resin (FR4), the materials most often applied in electronic industry for this purpose.
For the investigations, an immersion silver (iAg) double comb electrode structure was designed and prepared according to the IPC-B-24 standard. The iAg is still widely used in the electronics for surface finishes to protect the copper basic conductor.
The results of WD and THB tests were compared (see Fig 7-9.). Figure 7 shows that there are no significant differences between iAg on FR4 substrate and iAg on PI substrate and the average values are under 4 seconds.
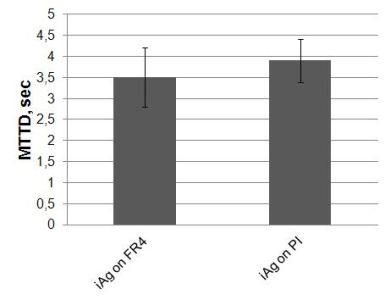
Figure 7. MTTD results of WD test on FR4 and PI substrates with iAg surface finishes (Failure criterion: 100 mVDC), without condensation effect (MTTF=MTTD).
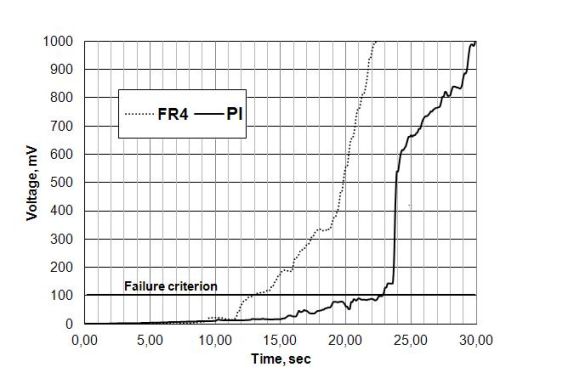
Figure 8. The MTTF difference between FR-4 and PI, with condensation effect.
In Fig. 8 a significant MTTF difference (~ 10 seconds) can be seen (see failure criterion), furthermore, the single values of FR4 and PI are also significantly higher compared to the WD test results (see Fig. 9).
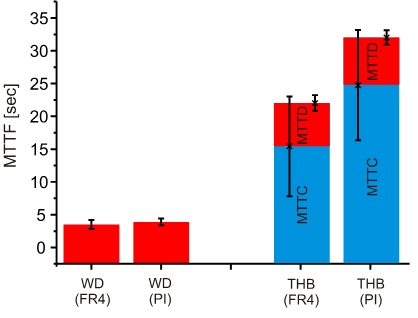
Figure 9. Comparison of WD and THB tests with regard to the condensation effect.
According to the results, the following can be stated:
With the adjustment of the above mentioned in situ and real time monitoring system, it can be concluded that MTTC has a significant impact on MTTF if the only difference is the insulation material (FR4 vs. PI) and the conductor material is iAg. Therefore, considering the above mentioned statements, the existing silver ECM model needs to be extended as follows (for more details, please see selected publications: b)):
MTTF = MTTC + MTTD
Another aim of my research work was to highlight some open questions and contradictions. A contradictory statement can be found about the comparison of the ECM behavior of copper and lead. Presently, an accepted ranking of the ECM susceptibility of silver, copper, tin and lead is the following: Ag>Pb>Cu>Sn (Silver is the worst). This ranking, which refers to time to failure was correlated with the different solubility product constant of different metal hydroxides (e.g. Sn(OH)2). The recent literature contains very different values about solubility product constants of lead(II)hydroxide. In addition, not pure Pb was used (Pb bearing solder alloy) in the experiments [9]. Therefore control investigations were needed and these found a significant difference between copper and lead bearing surface finishes (see Fig. 10), which latter was very similar to the Pb bearing solder alloy used in [9].

Figure 10. Significant ECM difference between copper and lead bearing surface finish (HASL).
According to the results, the following can be stated:
I have shown that the ranking position of copper and lead are not obvious, the ranking reported earlier (Ag>Pb>Cu>Sn), correlated with metal hydroxides [9]) should be modified to: Ag>Cu>Pb>Sn. In the case of lead-bearing solder alloy, the explanation is based on the formation of eutectic alloy and the modified anodic dissolution rate. In the case of copper, the oxidation state of the surface influences the dissolution (for more details, please see selected publications: c)).
Finally, I have carried out WD tests on low silver content, micro alloyed solder alloys, which are novel soldering materials. The MTTD was determined, and the structures of dendrites and their composition were investigated. Furthermore, the oxides formed on the anode surfaces were studied by XPS analysis as well. The WD test and XPS results were in good agreement and showed that some lead-free micro-alloyed low Ag content solders (e.g.: SAC0807) may pose high reliability risks in electronic devices due to their relative high electrochemical corrosion rate. (for more details, please see selected publications: d)).
Expected impact and further research
The results provide extended information about the ECM phenomenon. This is useful for a better understanding of the entire ECM process and thus, for creating an even more realistic model, which ensures improved reliability in electronics design.
Furthermore, I expect that the results will be useful for the electrical industry in choosing the proper materials and in setting up the optimal technological parameters during manufacturing as well. Some of the results are already being utilized by Robert Bosch GmbH in Stuttgart and by Continental Automotive Hungary Kft.
This work is connected to the scientific program titled "Development of quality-oriented and harmonized R+D+I strategy and functional model at BME", Project ID: TÁMOP-4.2.1/B-09/1/KMR-2010-0002 – NNA and the „Talent care and cultivation in the scientific workshops of BME" project, which is supported by the grant TÁMOP-4.2.2.B-10/1--2010-0009. Furthermore, the work supports the aims of the Campus Hungary Program as well. Nowadays, I am working at the National Institute for Materials Science (NIMS) in Japan (for 7 months), engaged in electrochemical migration investigations.
Publications, references, links
Selected Publications:
d)Bálint Medgyes, Balázs Illés, Gábor Harsányi, Electrochemical Migration Behaviour of Micro-alloyed Low Ag Content Solders in NaCl Solution. PERIODICA POLYTECHNICA-ELECTRICAL ENGINEERING, accepted for publication
Medgyes B, Illés B, Gál L, Measurements of Water Contact Angle on FR4 and Polyimide Substrates relating Electrochemical Migration. In: 36th International Spring Seminar on Electronics Technology, ISSE. Alba Iulia, Romania, 2013.05.08-2013.05.12. IEEE, pp. 1-4.
All publications:
Bálint Medgyes's List of Publications
References
[1] Directive of the European Commission for the Reduction of Hazardous Substances. Directive 2000/0159 (COD) C5-487/2002, LEX 391, PE-CONS 3662/2/02 Rev 2, ENV581, CODEC 1273, 2003.
[2] D. Q. Yu, W. Jillek, E. Schmitt, Electrochemical migration of lead free solder joints. Journal of Materials Science - Materials in Electronics. 17, 229-241 (2006)
[3] J.Y Jung, S.B. Lee, H.Y. Lee, Electrochemical Migration Characteristics of Eutectic Sn-Pb Solder Alloy in NaCl and Na2SO4 Solutions. Journal of Electronic Materials. 38, 691-699 (2009)
[4] G. Harsányi, Electrochemical Processes Resulting in Migrated Short Failures in Microcircuits. IEEE Transactions on Components, Packaging, and Manufacturing Technology-Part A, 18(3), pp. 602-610 (1995)
[5] B.I. Noh, J. W. Yoon, W.S. Hong, Evaluation of Electrochemical Migration on Flexible Printed Circuit Boards with Different Surface Finishes. Journal of Electronic Materials. 38, 902-907 (2009)
[6] D. Minzari at al, Electrochemical migration of tin in electronics and microstructure of the dendrits. Corrosion Science 53, 1659–1669 (2011)
[7] S. Yang et al, Initial Stage of Silver Electrochemical Migration Degradation. Microelectronics Reliability 46, 1915–1921 (2006)
[8] Z. Sheng, M.H. Azarian, M. Pecht, Reliability of Printed Circuit Boards Processed Using No-Clean Flux Technology in Temperature Humidity Bias Conditions Device and Materials Reliability. IEEE Transactions on Device and Materials Reliability. 8, 426-434 (2008)
[9] G. Harsányi and G. Inzelt, Comparing Migratory Resistive Short Formation Abilities of Conductor Systems Applied in Advanced Interconnection Systems. Microelectronics Reliability 41, (2001)
[10] D. Q.Yu, W. Jillek, E. Schmitt, Electrochemical migration of Sn-Pb and lead free solder alloys under distilled water. Journal of Materials Science - Materials in Electronics, 17, 219-227 (2006)
[11] S. J. Krumbein, Tutorial: Electrolytic Models for Metallic Electromigration Failure Mechanisms. IEEE Transactions on Reliability, 44( 4), pp. 539-549 (I995)
