 |
BMe Research Grant |

|
George Oláh Doctoral School
Department of Organic Chemistry and Technology
Supervisor: Prof. Péter Huszthy
Synthesis and molecular recognition studies of sensor and selector molecules containing a phenothiazine unit
Introducing the research area
Development of sensor and selector molecules capable of selective recognition and separation of ionic and neutral species or the enantiomers of chiral molecules is of great importance, for example in medical chemistry, environmental protection, pharmaceutical, food, cosmetic and pesticide industries.
Brief introduction of the research place
Our research group led by Prof. Péter Huszthy at the Department of Organic Chemistry and Technology aims to synthesize crown ethers and neutral anion sensors containing heterocyclic subunits. Further goal of our work is the molecular recognition studies of the newly synthesized derivatives.
History and context of the research
Selective complexation properties of sensor and selector molecules are based on molecular recognition, in which case the host molecule selectively binds a certain type of guest molecule through noncovalent bonding [1]. The phenomenon of molecular recognition is generally occurring in Nature. Now, I would like to draw attention to a widely known example, the natural ionophores (Figure 1).
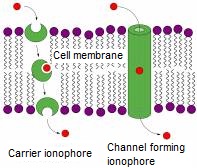
Figure 1: Natural ionophores
The first synthetic host molecules, the crown ethers were discovered in 1967 by Pedersen, who isolated dibenzo-18-crown-6 ether as a by-product [2]. He synthesized many cyclic polyethers and recognized that depending on their sizes, crown ethers could form complexes of different stability with chemically similar metal ions. The selectivity of crown ethers can be enhanced by increasing the rigidity of the macrocycle, which makes the host molecule preorganized for a certain guest molecule, meaning that the complex formation requires a small conformational change. The rigidity of the macrocycle can be achieved for example by incorporating a tricyclic heterocycle in the macroring. These derivatives can also be chromo- or fluorogenic, making them effective optical sensor molecules. Moreover, the properties of the donor atoms in the heterocycle can easily be modified by substitution in the aromatic rings.
There are numerous natural proteins that can bind anions via hydrogen bonding. For example, the sulfate binding protein (Figure 2) isolated from Salmonella Typhimurium performs the active transport of sulfate anion in the bacterium [3]. Based on the natural examples, there are many neutral anion sensor molecules reported in the literature, which bind anions through hydrogen bonding [4].
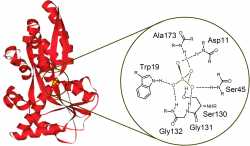
Figure 2: Sulfate binding protein
The research goal, open questions
During my previous work [A–D], we synthesized macrocycles containing acridone and acridine units, and studied their molecular recognition. In continuation of our research of crown ethers based on tricyclic ring systems, our attention turned toward phenothiazine. Our aim was to synthesize crown ethers containing a phenothiazine unit in which the NH group of the phenothiazine is part of the macroring. These crown ethers are good candidates for the selective complexation of biologically important metal ions, thus they can be used as sensor molecules. Furthermore, the properties of the nitrogen of phenothiazine can not only be modified by substitution in the aromatic rings, but also by changing the oxidation state of the sulfur atom making it a promising building element of new macrocycles with diverse applications.
During the synthesis of the precursors of the crown ethers we obtained several compounds, which are potential precursors of anion receptors. Further goal of my work was the synthesis of anion receptor molecules having a phenothiazine unit and also their molecular recognition studies by UV–vis spectroscopy.
Methodology
During the syntheses, preparative organic chemical methods were used. The progress of reactions was followed by thin layer chromatography. The crude products were purified by column chromatography, recrystallization or trituration. Purity of the compounds was determined by thin layer chromatography, measuring melting points and optical rotations. Structure of the products was determined using IR, NMR and MS spectroscopies and elemental analysis.
Complexation properties of the synthesized anion sensors were studied by UV–Vis spectroscopy in acetonitrile. During the titrations, a solution of the tetrabutylammonium salts of the anions (0.001 M, 0.01 M or 0.1 M) was added by Hamilton-syringe or automatic pipette to the solution of the receptor molecules. The concentrations of the solutions of the receptors during the titrations were 20 µM, 10 µM, 5 µM, 4 µM and 3.2 µM (we used more dilute solutions for the determination of higher stability constants). The measurements were performed in quartz cuvettes with path length of 1 cm (2.5 ml) in the case of 20 µM and 4 cm (10 ml) in the cases of 3.2–10 µM. Complex stability constants were determined by global nonlinear regression analysis using SPECFIT/32™ program based on the data of UV–Vis spectroscopic titrations.
Results
We worked out a new pathway for the synthesis of 1,9-disubstituted phenothiazines by nitration of 3,7-di-tert-butylphenothiazine followed by the transformation of the nitro groups. This way we synthesized 20 new 1,9-disubstituted phenothiazines, among them crown ethers (1–4, Figure 3) and anion sensors (5–9, Figure 3) containing a phenothiazine unit. [I–V].
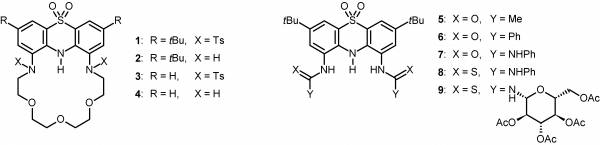
Figure 3: The new crown ethers and anion sensors
The anion recognition properties of the possible sensor molecules (5–9) were investigated with UV–Vis spectroscopy in acetonitrile. [I, II, IV, V].
The anion recognition ability of receptors 5 and 6 was studied toward the tetrabutylammonium salts of fluoride, chloride, bromide, iodide, hydrogen sulfate, dihydrogen phosphate and acetate. We demonstrated that the latter two derivatives do form complexes with most of the anions. In the cases of fluoride, acetate and dihydrogen phosphate, we observed the deprotonation of both sensor molecules. Formation of a simple hydrogen bonded complex could only be observed in the case of diacetamide 5 in the presence of chloride (Figure 4). The acetate induced deprotonation could be suppressed by acetic acid with promoting the complex formation. [I, II].
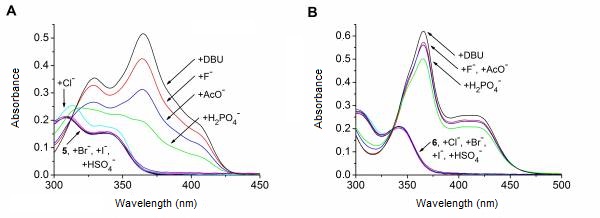
Figure 4: UV–Vis spectral changes of 5 (A) and 6 (B) upon addition of anions
As acetamide 5 and benzamide 6 did not form complexes with most of the anions, we synthesized urea 7 and thiourea 8 having more binding sites. The anion recognition ability of the latter two receptors was studied toward the tetrabutylammonium salts of fluoride, chloride, bromide, iodide, nitrate, hydrogen sulfate, sulfate, dihydrogen phosphate and acetate. Both sensor molecules formed hydrogen bonded complexes with most of the studied anions [II, IV].
Upon addition of one equivalent of fluoride or acetate to the solution of either receptor molecule (7 and 8), complexation induced changes could be observed in the absorption spectra. Further addition of these anions caused spectral changes characteristic to deprotonation, however, these spectra were different from those of the deprotonated receptors 7 and 8. These results suggested that deprotonated receptor–anion complexes had been formed (Figure 5) [II, IV].
X-ray crystallographic analysis of a single crystal grown from a solution of receptor 7 and three equivalents of tetrabutylammonium fluoride in an acetonitrile–hexane mixture confirmed that the deprotonated receptor complexed a fluoride anion by hydrogen bonds (Figure 5). [II, IV].
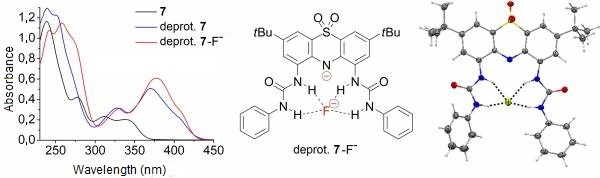
Figure 5: The fluoride complex of deprotonated 7
Bis(thiourea) 9 containing tetraacetylglucopyranosyl units had moderate enantioselectivity toward the enantiomers of the tetrabutylammonium salts of mandelic acid, some tert-butoxycarbonyl protected amino acids (phenylglycine, phenylalanine, alanine) and phenylglycine derivatives having other protecting groups (formyl, acetyl and pivaloyl protected derivatives). The enantioselectivity was Δ log K = 0.22 in the case of tert-butoxycarbonyl protected phenylglycine [II, V].
Expected impact and further research
By alkylating or acylating the secondary amino groups of crown ethers 2 and 4 new sensor and selector molecules can be synthesized for diverse applications. We would like to synthesize further derivatives of macrocycles 2 and 4 and study their molecular recognition.
The results obtained by the investigation of bis(phenylurea) 7 and bis(phenylthiourea) 8 suggest that anion receptors of similar structures with more acidic properties could provide an opportunity for the development of selective sensor molecules toward fluoride in basic media.
Publications, references, links
Publications related to the PhD thesis
- Kormos, A.; Móczár, I.; Sveiczer, A.; Baranyai, P.; Párkányi, L.; Tóth, K.; Huszthy, P. Tetrahedron 2012, 68, 7063–7069
- Kormos, A.; Huszthy, P. Magyar Kémiai Folyóirat, 2013, accepted for publication
- Kormos, A.; Sveiczer, A.; Fődi, T.; Rohonczi, Á.; Huszthy, P. Arkivoc, 2013, accepted for publication
- Kormos, A.; Móczár, I.; Pál, D.; Baranyai, P.; Holczbauer, T.; Palló, A.; Tóth, K.; Huszthy, P. Tetrahedron, sent back to the journal with required modifications
- Kormos, A.; Móczár, I.; Pál, D.; Baranyai, P.; Kupai, J.; Tóth, K.; Huszthy, P. Tetrahedron: Asymmetry 2013, 24, 62–65
Other publications
- Kertész, J.; Huszthy, P.; Kormos, A.; Bertha, F.; Horváth, V.; Horvai, G. Tetrahedron: Asymmetry 2009, 20, 2795–2801
- Kertész, J.; Móczár, I.; Kormos, A.; Baranyai, P.; Kubinyi, M.; Tóth, K.; Huszthy, P. Tetrahedron: Asymmetry 2011, 22, 684–689
- Kertész, J.; Huszthy, P.; Kormos, A.; Bezúr, L. Tetrahedron 2011, 67, 5206–5212
- Kertész, J.; Bognár, B.; Kormos, A.; Móczár, I.; Baranyai, P.; Kubinyi, M.; Kálai, T.; Hideg, K.; Huszthy, P. Tetrahedron 2011, 67, 8860–8864
Links
References
- Steed, J. W.; Atwood, J. L. Supramolecular Chemistry 2009, Wiley, 2nd ed.
- Pedersen, C. J. J. Am. Chem. Soc. 1967, 89, 2495–2496; 7017–7036
- Pflugrath, J. W.; Quiocho, F. A. J. Mol. Biol. 1988, 200, 163–180
- Wenzel, M.; Hiscock, J. R.; Gale, P. A. Chem. Soc. Rev. 2012, 41, 480–520
